Nogo-A expression in the brain of mice with cerebral malaria
- PMID: 21980529
- PMCID: PMC3183069
- DOI: 10.1371/journal.pone.0025728
Nogo-A expression in the brain of mice with cerebral malaria
Abstract
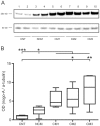
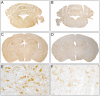
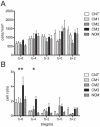
Cerebral malaria (CM) is associated with a high rate of transient or persistent neurological sequelae. Nogo-A, a protein that is highly expressed in the endoplasmic reticulum (ER) of the mammalian central nervous system (CNS), is involved in neuronal regeneration and synaptic plasticity in the injured CNS. The current study investigates the role of Nogo-A in the course of experimental CM. C57BL/6J mice were infected with Plasmodium berghei ANKA blood stages. Brain homogenates of mice with different clinical severity levels of CM, infected animals without CM and control animals were analyzed for Nogo-A up-regulation by Western blotting and immunohistochemistry. Brain regions with Nogo-A upregulation were evaluated by transmission electron microscopy. Densitometric analysis of Western blots yielded a statistically significant upregulation of Nogo-A in mice showing moderate to severe CM. The number of neurons and oligodendrocytes positive for Nogo-A did not differ significantly between the studied groups. However, mice with severe CM showed a significantly higher number of cells with intense Nogo-A staining in the brain stem. In this region ultrastructural alterations of the ER were regularly observed. Nogo-A is upregulated during the early course of experimental CM. In the brain stem of severely affected animals increased Nogo-A expression and ultrastructural changes of the ER were observed. These data indicate a role of Nogo-A in neuronal stress response during experimental CM.
Conflict of interest statement
Figures


Similar articles
-
Complement factors C1q, C3 and C5 in brain and serum of mice with cerebral malaria.Malar J. 2008 Oct 10;7:207. doi: 10.1186/1475-2875-7-207. Malar J. 2008. PMID: 18847493 Free PMC article.
-
Apoptosis in experimental cerebral malaria: spatial profile of cleaved caspase-3 and ultrastructural alterations in different disease stages.Neuropathol Appl Neurobiol. 2007 Oct;33(5):560-71. doi: 10.1111/j.1365-2990.2007.00833.x. Epub 2007 Apr 18. Neuropathol Appl Neurobiol. 2007. PMID: 17442059
-
Brain-derived neurotrophic factor and the course of experimental cerebral malaria.Brain Res. 2013 Jan 15;1490:210-24. doi: 10.1016/j.brainres.2012.10.040. Epub 2012 Nov 1. Brain Res. 2013. PMID: 23123703
-
Cell autonomous function of Nogo and reticulons: The emerging story at the endoplasmic reticulum.J Cell Physiol. 2008 Aug;216(2):303-8. doi: 10.1002/jcp.21434. J Cell Physiol. 2008. PMID: 18330888 Review.
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
Cited by
-
Plasma and cerebrospinal proteomes from children with cerebral malaria differ from those of children with other encephalopathies.J Infect Dis. 2013 Nov 1;208(9):1494-503. doi: 10.1093/infdis/jit334. Epub 2013 Jul 25. J Infect Dis. 2013. PMID: 23888081 Free PMC article.
References
-
- Schmutzhard E, Gerstenbrand F. Cerebral malaria in Tanzania. Its epidemiology, clinical symptoms and neurological long term sequelae in the light of 66 cases. Trans R Soc Trop Med Hyg. 1984;78:351–353. - PubMed
-
- Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. - PubMed
-
- Marchiafava E, Bignami A. Malaria and the Parasites of Malaria Fevers. London: The New Sydenham Society; 1894. On Summer-Autumnal malaria fevers. pp. 1–234.
-
- Clark H, Tomlinson W. The pathological anatomy of malaria. In: Boyd M, editor. Malariology. Philadelphia: W. B. Saunders; 1945. pp. 874–903.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

