Scabies mite peritrophins are potential targets of human host innate immunity
- PMID: 21980545
- PMCID: PMC3181238
- DOI: 10.1371/journal.pntd.0001331
Scabies mite peritrophins are potential targets of human host innate immunity
Erratum in
-
Correction: Scabies Mite Peritrophins Are Potential Targets of Human Host Innate Immunity.PLoS Negl Trop Dis. 2024 Jul 11;18(7):e0012329. doi: 10.1371/journal.pntd.0012329. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 38990803 Free PMC article.
Abstract
Background: Pruritic scabies lesions caused by Sarcoptes scabiei burrowing in the stratum corneum of human skin facilitate opportunistic bacterial infections. Emerging resistance to current therapeutics emphasizes the need to identify novel targets for protective intervention. We have characterized several protein families located in the mite gut as crucial factors for host-parasite interactions. Among these multiple proteins inhibit human complement, presumably to avoid complement-mediated damage of gut epithelial cells. Peritrophins are major components of the peritrophic matrix often found in the gut of arthropods. We hypothesized that a peritrophin, if abundant in the scabies mite gut, could be an activator of complement.
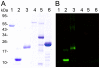
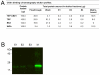
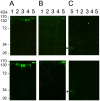
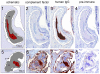
Methodology/principal findings: A novel full length scabies mite peritrophin (SsPTP1) was identified in a cDNA library from scabies mites. The amino acid sequence revealed four putative chitin binding domains (CBD). Recombinant expression of one CBD of the highly repetitive SsPTP1 sequence as TSP-hexaHis-fusion protein resulted in soluble protein, which demonstrated chitin binding activity in affinity chromatography assays. Antibodies against a recombinant SsPTP1 fragment were used to immunohistochemically localize native SsPTP1 in the mite gut and in fecal pellets within the upper epidermis, co-localizing with serum components such as host IgG and complement. Enzymatic deglycosylation confirmed strong N- and O-glycosylation of the native peritrophin. Serum incubation followed by immunoblotting with a monoclonal antibody against mannan binding lectin (MBL), the recognition molecule of the lectin pathway of human complement activation, indicated that MBL may specifically bind to glycosylated SsPTP1.
Conclusions/significance: This study adds a new aspect to the accumulating evidence that complement plays a major role in scabies mite biology. It identifies a novel peritrophin localized in the mite gut as a potential target of the lectin pathway of the complement cascade. These initial findings indicate a novel role of scabies mite peritrophins in triggering a host innate immune response within the mite gut.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Hengge UR, Currie BJ, Jäger G, Lupi O, Schwartz RA. Scabies: a ubiquitous neglected skin disease. The Lancet Infectious Diseases. 2006;6:769–779. - PubMed
-
- Arlian LG. Biology, host relations and epidemiology of Sarcoptes scabiei. Annual Review of Entomology. 1989;34:139–161. - PubMed
-
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35:197–206.
-
- Walton SF, Holt DC, Currie BJ, Kemp DJ, Baker, et al. Advances in Parasitology: Academic Press; 2004. Scabies: New Future for a Neglected Disease. pp. 309–376. - PubMed
-
- Currie BJ, Carapetis JR. Skin infections and infestations in Aboriginal communities in northern Australia. Australas J Dermatol. 2000;41:139–143; quiz 144–135. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

