A net-like structure with pores is observed during cell fusion induced by the receptor FGFRL1
- PMID: 21980560
- PMCID: PMC3187888
- DOI: 10.4161/cib.4.3.14892
A net-like structure with pores is observed during cell fusion induced by the receptor FGFRL1
Abstract
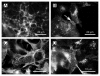
FGFRL1 is the fifth member of the fibroblast growth factor receptor (FGFR) family. Similar to the other members, it harbors three Ig loops in its extracellular domain, but in contrast to the other receptors, it lacks the intracellular protein tyrosine kinase domain that would be required for signaling by transphosphorylation. FGFRL1 is mainly found in the musculoskeletal system, where it appears to inhibit cell proliferation but to induce cell adhesion and differentiation. Mice with a targeted disruption of the FGFRL1 gene die during birth due to a malformed diaphragm muscle, which is not strong enough to inflate the lungs after birth. Expression of FGFRL1 is highly upregulated during the differentiation of myoblasts to multinucleated myotubes, suggesting an important role for FGFRL1 in cell-cell fusion. Recently we showed that FGFRL1 does indeed induce fusion of cultured cells into large syncytia. A reporter gene assay demonstrated that the third Ig domain and the transmembrane domain of FGFRL1 are both necessary and sufficient to fuse CHO cells into syncytia comprising several hundred nuclei. At the contact site, the fusing cells reveal a peculiar net-like structure with pores of about 1 µm diameter. It is possible that these structures represent membrane areas with fusion pores that set in motion the cell-cell fusion process. FGFRL1 is the first mammalian protein that is capable of triggering cell-cell fusion in vitro.
Keywords: CD9; FGFRL1; cell-cell fusion; fibroblast growth factor (FGF); fibroblast growth factor receptor (FGFR); fusion pores; muscle formation.
Figures

Comment on
-
Rapid fusion and syncytium formation of heterologous cells upon expression of the FGFRL1 receptor.J Biol Chem. 2010 Nov 26;285(48):37704-15. doi: 10.1074/jbc.M110.140517. Epub 2010 Sep 17. J Biol Chem. 2010. PMID: 20851884 Free PMC article.
Similar articles
-
Rapid fusion and syncytium formation of heterologous cells upon expression of the FGFRL1 receptor.J Biol Chem. 2010 Nov 26;285(48):37704-15. doi: 10.1074/jbc.M110.140517. Epub 2010 Sep 17. J Biol Chem. 2010. PMID: 20851884 Free PMC article.
-
The FGFRL1 receptor is shed from cell membranes, binds fibroblast growth factors (FGFs), and antagonizes FGF signaling in Xenopus embryos.J Biol Chem. 2010 Jan 15;285(3):2193-202. doi: 10.1074/jbc.M109.058248. Epub 2009 Nov 17. J Biol Chem. 2010. PMID: 19920134 Free PMC article.
-
Targeted disruption of the intracellular domain of receptor FgfrL1 in mice.PLoS One. 2014 Aug 15;9(8):e105210. doi: 10.1371/journal.pone.0105210. eCollection 2014. PLoS One. 2014. PMID: 25126760 Free PMC article.
-
Biology of FGFRL1, the fifth fibroblast growth factor receptor.Cell Mol Life Sci. 2011 Mar;68(6):951-64. doi: 10.1007/s00018-010-0576-3. Epub 2010 Nov 16. Cell Mol Life Sci. 2011. PMID: 21080029 Free PMC article. Review.
-
Comprehending fibroblast growth factor receptor like 1: Oncogene or tumor suppressor?Cancer Treat Res Commun. 2021;29:100472. doi: 10.1016/j.ctarc.2021.100472. Epub 2021 Oct 5. Cancer Treat Res Commun. 2021. PMID: 34689016 Review.
Cited by
-
FGFRL1: Structure, Molecular Function, and Involvement in Human Disease.Curr Issues Mol Biol. 2025 Apr 17;47(4):286. doi: 10.3390/cimb47040286. Curr Issues Mol Biol. 2025. PMID: 40699684 Free PMC article. Review.
References
-
- Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. - PubMed
-
- Oren-Suissa M, Podbilewicz B. Cell fusion during development. Trends Cell Biol. 2007;17:537–546. - PubMed
-
- Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. - PubMed
-
- Abmayr SM, Zhuang S, Geisbrecht ER. Myoblast fusion in Drosophila. Methods Mol Biol. 2008;475:75–97. - PubMed
-
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. - PubMed
LinkOut - more resources
Full Text Sources
