The immunity-related GTPase Irgm3 relieves endoplasmic reticulum stress response during coxsackievirus B3 infection via a PI3K/Akt dependent pathway
- PMID: 21981022
- PMCID: PMC3691006
- DOI: 10.1111/j.1462-5822.2011.01708.x
The immunity-related GTPase Irgm3 relieves endoplasmic reticulum stress response during coxsackievirus B3 infection via a PI3K/Akt dependent pathway
Abstract
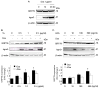
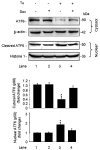
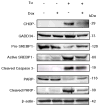
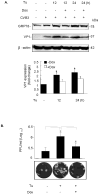
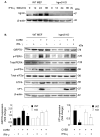
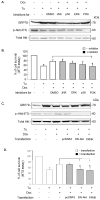
The IRG protein Irgm3 preserves cell survival during coxsackievirus B3 (CVB3) infection. However, the molecular mechanisms are not clear. Here, we examined the effect of Irgm3 expression on ER stress triggered by pharmacological agents or CVB3 infection. In Tet-On/Irgm3 HeLa cells, Irgm3 expression suppressed either chemical- or CVB3-induced upregulation of glucose-regulated protein 78. Further, Irgm3 strongly inhibited the activation of both the PERK and ATF6 pathways of ER stress responses, which further led to the diminished phosphorylation of eIF2α, reduced cleavage/activation of transcription factor SREBP1 and attenuated induction of proapoptotic genes CHOP and GADD34. These data were further supported by experiments using Irgm3 knockout mouse embryonic fibroblasts, in which the ER stress induced by CVB3 was not relieved due to the lack of Irgm3 expression. In addition, the tunicamycin-triggered ER stress promoted the subsequent CVB3 infection. The effect of Irgm3 on ER stress and CVB3 infection was diminished by the PI3K inhibitor, LY294002, while inhibitors of ERK, JNK and p38 had no effect. These data were further corroborated by transfection of cells with a dominant negative Akt. Taken together, these data suggest that Irgm3 relieves the ER stress response via a PI3K/Akt dependent mechanism, which contributes to host defence against CVB3 infection.
© 2011 Blackwell Publishing Ltd.
Figures






References
-
- Alwine JC. Modulation of host cell stress responses by human cytomegalovirus. Curr Top Microbiol Immunol. 2008;325:263–279. - PubMed
-
- Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem. 2004;279:5288–5297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

