RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts
- PMID: 21981915
- PMCID: PMC3192321
- DOI: 10.1016/j.molcel.2011.09.003
RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts
Abstract
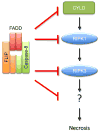
Caspase-8, FADD, and FLIP orchestrate apoptosis in response to death receptor ligation. Mysteriously however, these proteins are also required for normal embryonic development and immune cell proliferation, an observation that has led to their implication in several nonapoptotic processes. While many scenarios have been proposed, recent genetic and biochemical evidence points to unregulated signaling by the receptor-interacting protein kinases-1 (RIPK1) and RIPK3 as the lethal defect in caspase-8-, FADD-, and FLIP-deficient animals and tissues. The RIPKs are known killers, being responsible for a nonapoptotic form of cell death with features similar to necrosis. However, the mechanism by which caspase-8, FADD, and FLIP prevent runaway RIPK activation is unknown, and the signals that trigger these events during development and immune cell activation remain at large. In this review, we will lay out the evidence as it now stands, reinterpreting earlier observations in light of new clues and considering where the investigation might lead.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Alappat EC, Feig C, Boyerinas B, Volkland J, Samuels M, Murmann AE, Thorburn A, Kidd VJ, Slaughter CA, Osborn SL, et al. Phosphorylation of FADD at serine 194 by CKIalpha regulates its nonapoptotic activities. Mol Cell. 2005;19:321–332. - PubMed
-
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. - PubMed
-
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

