A novel method for spectrophotometric determination of pregabalin in pure form and in capsules
- PMID: 21982305
- PMCID: PMC3238223
- DOI: 10.1186/1752-153X-5-59
A novel method for spectrophotometric determination of pregabalin in pure form and in capsules
Abstract
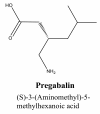
Background: Pregabalin, a γ-amino-n-butyric acid derivative, is an antiepileptic drug not yet official in any pharmacopeia and development of analytical procedures for this drug in bulk/formulation forms is a necessity. We herein, report a new, simple, extraction free, cost effective, sensitive and reproducible spectrophotometric method for the determination of the pregabalin.
Results: Pregabalin, as a primary amine was reacted with ninhydrin in phosphate buffer pH 7.4 to form blue violet colored chromogen which could be measured spectrophotometrically at λmax 402.6 nm. The method was validated with respect to linearity, accuracy, precision and robustness. The method showed linearity in a wide concentration range of 50-1000 μg mL-1 with good correlation coefficient (0.992). The limits of assays detection was found to be 6.0 μg mL-1 and quantitation limit was 20.0 μg mL-1. The suggested method was applied to the determination of the drug in capsules. No interference could be observed from the additives in the capsules. The percentage recovery was found to be 100.43 ± 1.24.
Conclusion: The developed method was successfully validated and applied to the determination of pregabalin in bulk and pharmaceutical formulations without any interference from common excipients. Hence, this method can be potentially useful for routine laboratory analysis of pregabalin.
Figures




References
-
- Vaidya VV, Yetal SM, Roy SMN, Gomes NA, Joshi SS. LC-MS-MS Determination of Pregabalin in Human Plasma. Chromatographia. 2007;66(11-12):925–928. doi: 10.1365/s10337-007-0430-4. - DOI
-
- Mandal U, Sarkar AK, Gowda KV, Agarwal S, Bose A, Bhaumik U, Ghosh D, Pal TK. Determination of Pregabalin in Human Plasma Using LC-MS-MS. Chromatographia. 2008;67(3-4):237–243. doi: 10.1365/s10337-007-0440-2. - DOI
LinkOut - more resources
Full Text Sources

