INSPIRE: A phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer
- PMID: 21982342
- PMCID: PMC3203100
- DOI: 10.1186/1471-2407-11-430
INSPIRE: A phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer
Abstract
Background: Previous research suggests the therapeutic cancer vaccine L-BLP25 potentially provides a survival benefit in patients with locally advanced unresectable stage III non-small cell lung carcinoma (NSCLC). These promising findings prompted the phase III study, INSPIRE, in patients of East-Asian ethnicity. East-Asian ethnicity is an independent favourable prognostic factor for survival in NSCLC. The favourable prognosis is most likely due to a higher incidence of EGFR mutations among this patient population.
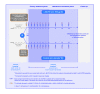
Methods/design: The primary objective of the INSPIRE study is to assess the treatment effect of L-BLP25 plus best supportive care (BSC), as compared to placebo plus BSC, on overall survival time in East-Asian patients with unresectable stage III NSCLC and either documented stable disease or an objective response according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria following primary chemoradiotherapy. Those in the L-BLP25 arm will receive a single intravenous infusion of cyclophosphamide (300 mg/m2) 3 days before the first L-BLP25 vaccination, with a corresponding intravenous infusion of saline to be given in the control arm. A primary treatment phase of 8 subcutaneous vaccinations of L-BLP25 930 μg or placebo at weekly intervals will be followed by a maintenance treatment phase of 6-weekly vaccinations continued until disease progression or discontinuation from the study.
Discussion: The ongoing INSPIRE study is the first large study of a therapeutic cancer vaccine specifically in an East-Asian population. It evaluates the potential of maintenance therapy with L-BLP25 to prolong survival in East-Asian patients with stage III NSCLC where there are limited treatment options currently available. STUDY NUMBER: EMR 63325-012
Trial registration: Clinicaltrials.gov reference: NCT01015443.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous