Protease regulation: the Yin and Yang of neural development and disease
- PMID: 21982365
- PMCID: PMC3221598
- DOI: 10.1016/j.neuron.2011.09.012
Protease regulation: the Yin and Yang of neural development and disease
Abstract
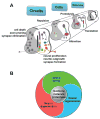
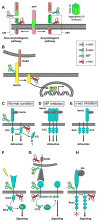
The formation, maintenance, and plasticity of neural circuits rely upon a complex interplay between progressive and regressive events. Increasingly, new functions are being identified for axon guidance molecules in the dynamic processes that occur within the embryonic and adult nervous system. The magnitude, duration, and spatial activity of axon guidance molecule signaling are precisely regulated by a variety of molecular mechanisms. Here we focus on recent progress in understanding the role of protease-mediated cleavage of guidance factors required for directional axon growth, with a particular emphasis on the role of metalloprotease and γ-secretase. Since axon guidance molecules have also been linked to neural degeneration and regeneration in adults, studies of guidance receptor proteolysis are beginning to define new relationships between neurodevelopment and neurodegeneration. These findings raise the possibility that the signaling checkpoints controlled by proteases could be useful targets to enhance regeneration.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A. Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol Cell Neurosci. 2005;28:64–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

