West Nile virus infection does not induce PKR activation in rodent cells
- PMID: 21982595
- PMCID: PMC3208726
- DOI: 10.1016/j.virol.2011.08.008
West Nile virus infection does not induce PKR activation in rodent cells
Abstract
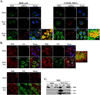
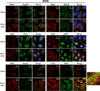
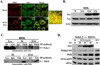
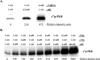
dsRNA-activated protein kinase (PKR) is activated by viral dsRNAs and phosphorylates eIF2a reducing translation of host and viral mRNA. Although infection with a chimeric West Nile virus (WNV) efficiently induced PKR and eIF2a phosphorylation, infections with natural lineage 1 or 2 strains did not. Investigation of the mechanism of suppression showed that among the cellular PKR inhibitor proteins tested, only Nck, known to interact with inactive PKR, colocalized and co-immunoprecipitated with PKR in WNV-infected cells and PKR phosphorylation did not increase in infected Nck1,2-/- cells. Several WNV stem-loop RNAs efficiently activated PKR in vitro but not in infected cells. WNV infection did not interfere with intracellular PKR activation by poly(I:C) and similar virus yields were produced by control and PKR-/- cells. The results indicate that PKR phosphorylation is not actively suppressed in WNV-infected cells but that PKR is not activated by the viral dsRNA in infected cells.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. - PubMed
-
- Cardin E, Larose L. Nck-1 interacts with PKR and modulates its activation by dsRNA. Biochem Biophys Res Commun. 2008;377:231–235. - PubMed
-
- Cardin E, Latreille M, Khoury C, Greenwood MT, Larose L. Nck-1 selectively modulates eIF2alphaSer51 phosphorylation by a subset of eIF2alpha-kinases. FEBS J. 2007;274:5865–5875. - PubMed
-
- Conn GL. Expression of active RNA-activated protein kinase (PKR) in bacteria. Biotechniques. 2003;35:682–684. 686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

