Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation
- PMID: 21982596
- PMCID: PMC3205267
- DOI: 10.1016/j.immuni.2011.08.001
Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation
Erratum in
- Immunity. 2011 Oct 28;35(4):649
Abstract
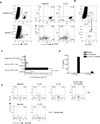
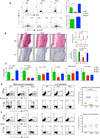
Interleukin-23 (IL-23) and CD4(+) T helper 17 (Th17) cells are thought to be critical in psoriasis pathogenesis. Here, we report that IL-23 predominantly stimulated dermal γδ T cells to produce IL-17 that led to disease progression. Dermal γδ T cells constitutively expressed the IL-23 receptor (IL-23R) and transcriptional factor RORγt. IL-17 production from dermal γδ T cells was independent of αβ T cells. The epidermal hyperplasia and inflammation induced by IL-23 were significantly decreased in T cell receptor δ-deficient (Tcrd(-/-)) and IL-17 receptor-deficient (Il17ra(-/-)) mice but occurred normally in Tcra(-/-) mice. Imiquimod-induced skin pathology was also significantly decreased in Tcrd(-/-) mice. Perhaps further promoting disease progression, IL-23 stimulated dermal γδ T cell expansion. In psoriasis patients, γδ T cells were greatly increased in affected skin and produced large amounts of IL-17. Thus, IL-23-responsive dermal γδ T cells are the major IL-17 producers in the skin and may represent a novel target for the treatment of psoriasis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Inflammation: Under the skin.Nat Rev Immunol. 2011 Nov 4;11(12):800. doi: 10.1038/nri3113. Nat Rev Immunol. 2011. PMID: 22051889 No abstract available.
References
-
- Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur J Immunol. 2009;39:1221–1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

