The arrestin domain-containing 3 protein regulates body mass and energy expenditure
- PMID: 21982743
- PMCID: PMC3216113
- DOI: 10.1016/j.cmet.2011.08.011
The arrestin domain-containing 3 protein regulates body mass and energy expenditure
Abstract
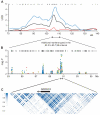
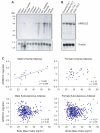
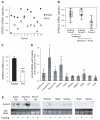
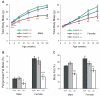
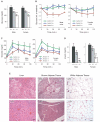
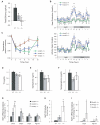
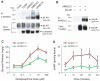
A human genome-wide linkage scan for obesity identified a linkage peak on chromosome 5q13-15. Positional cloning revealed an association of a rare haplotype to high body-mass index (BMI) in males but not females. The risk locus contains a single gene, "arrestin domain-containing 3" (ARRDC3), an uncharacterized α-arrestin. Inactivating Arrdc3 in mice led to a striking resistance to obesity, with greater impact on male mice. Mice with decreased ARRDC3 levels were protected from obesity due to increased energy expenditure through increased activity levels and increased thermogenesis of both brown and white adipose tissues. ARRDC3 interacted directly with β-adrenergic receptors, and loss of ARRDC3 increased the response to β-adrenergic stimulation in isolated adipose tissue. These results demonstrate that ARRDC3 is a gender-sensitive regulator of obesity and energy expenditure and reveal a surprising diversity for arrestin family protein functions.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1323–1332. - PubMed
-
- Bell CG, Benzinou M, Siddiq A, Lecoeur C, Dina C, Lemainque A, Clément K, Basdevant A, Guy-Grand B, Mein CA, et al. Genome-wide linkage analysis for severe obesity in french caucasians finds significant susceptibility locus on chromosome 19q. Diabetes. 2004;53:1857–1865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL103582/HL/NHLBI NIH HHS/United States
- HL090553/HL/NHLBI NIH HHS/United States
- R01 DK056626/DK/NIDDK NIH HHS/United States
- HL081523/HL/NHLBI NIH HHS/United States
- R01 DK076117/DK/NIDDK NIH HHS/United States
- P01 HL090553/HL/NHLBI NIH HHS/United States
- R01 DK048873/DK/NIDDK NIH HHS/United States
- R01 DK036256/DK/NIDDK NIH HHS/United States
- R01 HL103582/HL/NHLBI NIH HHS/United States
- HL048743/HL/NHLBI NIH HHS/United States
- K25 HL081523/HL/NHLBI NIH HHS/United States
- P01 HL048743/HL/NHLBI NIH HHS/United States
- DK36256/DK/NIDDK NIH HHS/United States
- R29 DK048873/DK/NIDDK NIH HHS/United States
- DK076117/DK/NIDDK NIH HHS/United States
- DK56626/DK/NIDDK NIH HHS/United States
- DK48873/DK/NIDDK NIH HHS/United States
- R37 DK048873/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

