Structural remodeling of astrocytes in the injured CNS
- PMID: 21982954
- PMCID: PMC3713769
- DOI: 10.1177/1073858411423441
Structural remodeling of astrocytes in the injured CNS
Abstract
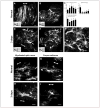
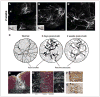
Astrocytes respond to all forms of CNS insult and disease by becoming reactive, a nonspecific but highly characteristic response that involves various morphological and molecular changes. Probably the most recognized aspect of reactive astrocytes is the formation of a glial scar that impedes axon regeneration. Although the reactive phenotype was first suggested more than 100 years ago based on morphological changes, the remodeling process is not well understood. We know little about the actual structure of a reactive astrocyte, how an astrocyte remodels during the progression of an insult, and how populations of these cells reorganize to form the glial scar. New methods of labeling astrocytes, along with transgenic mice, allow the complete morphology of reactive astrocytes to be visualized. Recent studies show that reactivity can induce a remarkable change in the shape of a single astrocyte, that not all astrocytes react in the same way, and that there is plasticity in the reactive response.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






References
-
- Allansson L, Khatibi S, Gustavsson T, Blomstrand F, Olsson T, Hansson E. Single-cell volume estimation by three-dimensional wide-field microscopy applied to astroglial primary cultures. J Neurosci Methods. 1999;93(1):1–11. - PubMed
-
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141(1):41–53. - PubMed
-
- Benesova J, Hock M, Butenko O, Prajerova I, Anderova M, Chvatal A. Quantification of astrocyte volume changes during ischemia in situ reveals two populations of astrocytes in the cortex of GFAP/EGFP mice. J Neurosci Res. 2009;87(1):96–111. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

