Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency
- PMID: 21983175
- PMCID: PMC3732466
- DOI: 10.1684/mrh.2011.0286
Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency
Abstract
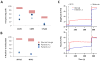
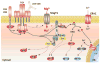
Although Mg(2+) has a well-recognized role as an essential cofactor for all ATP-binding enzymes, its role as a signaling ion, like Ca(2+), has been controversial. A requirement for Mg(2+)for optimal T lymphocyte stimulation was demonstrated more than 30 years ago, but the mechanism of its synergistic effect with Ca(2+)in T cell activation remains elusive. Here, we summarize our recent discovery of a signaling role for Mg(2+)in the T cell antigen receptor (TCR) signaling pathway from the study of a novel primary immunodeficiency, now named X-linked immunodeficiency with Mg(2+)defect, EBV infection and neoplasia (XMEN). XMEN patients were found to have a deficiency in magnesium transporter 1 (MAGT1), an Mg(2+)-specific transporter, which leads to the absence of a TCR-stimulated Mg(2+)flux and an attenuation of T cell activation. We further showed that this Mg(2+)flux is required proximally for the temporal orchestration of phospholipase C-γ1 (PLCγ1) activation. Thus, our study not only provides a second messenger role for Mg(2+)to explain its synergism with calcium in T cell signaling, it also identifies a potential extracellular therapeutic target for T cell-specific immunomodulation.
Figures
References
-
- Cakmak I, Kirkby EA. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol Plant. 2008;133(4):692–704. - PubMed
-
- Cowan JA. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals. 2002;15(3):225–35. - PubMed
-
- Yang W, Lee JY, Nowotny M. Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol Cell. 2006;22(1):5–13. - PubMed
-
- Murphy E. Mysteries of magnesium homeostasis. Circ Res. 2000;86(3):245–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
