The proteoglycan bikunin has a defined sequence
- PMID: 21983600
- PMCID: PMC3197799
- DOI: 10.1038/nchembio.673
The proteoglycan bikunin has a defined sequence
Abstract
Proteoglycans are complex glycoconjugates that regulate critical biological pathways in all higher organisms. Bikunin, the simplest proteoglycan, with a single glycosaminoglycan chain, is a serine protease inhibitor used to treat acute pancreatitis. Unlike nucleic acids and proteins, whose synthesis is template driven, Golgi-synthesized glycosaminoglycans are not believed to have predictable or deterministic sequences. Bikunin peptidoglycosaminoglycans were prepared and fractionated to obtain a collection of size-similar and charge-similar chains. Fourier transform mass spectral analysis identified a small number of parent molecular ions corresponding to monocompositional peptidoglycosaminoglycans. Fragmentation using collision-induced dissociation unexpectedly afforded a single sequence for each monocompositional parent ion, unequivocally demonstrating the presence of a defined sequence. The biosynthetic pathway common to all proteoglycans suggests that even more structurally complex proteoglycans, such as heparan sulfate, may have defined sequences, requiring a readjustment in the understanding of information storage in complex glycans.
Figures
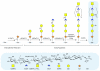
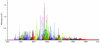
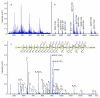

Comment in
-
Carbohydrates: Cracking the glycan sequence code.Nat Chem Biol. 2011 Oct 18;7(11):758-9. doi: 10.1038/nchembio.696. Nat Chem Biol. 2011. PMID: 22008993 No abstract available.
-
Sequencing for sugars.Nat Methods. 2011 Dec;8(12):996-7. doi: 10.1038/nmeth.1798. Nat Methods. 2011. PMID: 22238776 No abstract available.
References
-
- Feero WG, Guttmacher AE, Collins FS. Genomic medicine--an updated primer. N Engl J Med. 2010;362:2001–2011. - PubMed
-
- Ball S, et al. From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell. 1996;86:349–352. - PubMed
-
- Perez S, Mazeau K. Conformations, structures, and morphologies of celluloses. In: Dumitiu S, editor. Polysaccharides: Structural diversity and functional versatility. 2nd edn. Marcel Dekker; New York: 1998. pp. 41–68.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

