A crucial requirement for Hedgehog signaling in small cell lung cancer
- PMID: 21983857
- PMCID: PMC3380617
- DOI: 10.1038/nm.2473
A crucial requirement for Hedgehog signaling in small cell lung cancer
Abstract
Small-cell lung cancer (SCLC) is an aggressive neuroendocrine subtype of lung cancer for which there is no effective treatment. Using a mouse model in which deletion of Rb1 and Trp53 in the lung epithelium of adult mice induces SCLC, we found that the Hedgehog signaling pathway is activated in SCLC cells independently of the lung microenvironment. Constitutive activation of the Hedgehog signaling molecule Smoothened (Smo) promoted the clonogenicity of human SCLC in vitro and the initiation and progression of mouse SCLC in vivo. Reciprocally, deletion of Smo in Rb1 and Trp53-mutant lung epithelial cells strongly suppressed SCLC initiation and progression in mice. Furthermore, pharmacological blockade of Hedgehog signaling inhibited the growth of mouse and human SCLC, most notably following chemotherapy. These findings show a crucial cell-intrinsic role for Hedgehog signaling in the development and maintenance of SCLC and identify Hedgehog pathway inhibition as a therapeutic strategy to slow the progression of disease and delay cancer recurrence in individuals with SCLC.
Figures
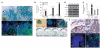
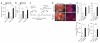


References
-
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113:5–21. - PubMed
-
- Meuwissen R, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. - PubMed
-
- Vestergaard J, et al. Hedgehog signaling in small-cell lung cancer: frequent in vivo but a rare event in vitro. Lung Cancer. 2006;52:281–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

