Crystal structures of the extracellular domain of LRP6 and its complex with DKK1
- PMID: 21984209
- PMCID: PMC3249237
- DOI: 10.1038/nsmb.2139
Crystal structures of the extracellular domain of LRP6 and its complex with DKK1
Abstract
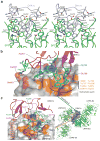
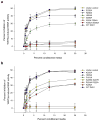
Low-density-lipoprotein (LDL) receptor-related proteins 5 and 6 (LRP5/6) are Wnt co-receptors essential for Wnt/β-catenin signaling. Dickkopf 1 (DKK1) inhibits Wnt signaling by interacting with the extracellular domains of LRP5/6 and is a drug target for multiple diseases. Here we present the crystal structures of a human LRP6-E3E4-DKK1 complex and the first and second halves of human LRP6's four propeller-epidermal growth factor (EGF) pairs (LRP6-E1E2 and LRP6-E3E4). Combined with EM analysis, these data demonstrate that LRP6-E1E2 and LRP6-E3E4 form two rigid structural blocks, with a short intervening hinge that restrains their relative orientation. The C-terminal domain of DKK1 (DKK1c) interacts with the top surface of the LRP6-E3 YWTD propeller and given their structural similarity, probably also that of the LRP6-E1 propeller, through conserved hydrophobic patches buttressed by a network of salt bridges and hydrogen bonds. Our work provides key insights for understanding LRP5/6 structure and the interaction of LRP5/6 with DKK, as well as for drug discovery.
Conflict of interest statement
R.T.M. is a cofounder of, and consultant with, FATE Therapeutics. The other authors declare no competing financial interests.
Figures




Comment in
-
Cell signaling. Crystallizing WNT signalling.Nat Rev Mol Cell Biol. 2012 Jan;13(1):4. doi: 10.1038/nrm3260. Nat Rev Mol Cell Biol. 2012. PMID: 22295277 No abstract available.
References
-
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. - PubMed
-
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–6. - PubMed
-
- Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

