Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis
- PMID: 21984241
- PMCID: PMC3233067
- DOI: 10.1128/AEM.05151-11
Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis
Abstract
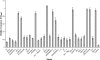
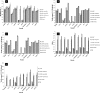
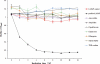
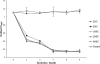
Bacterial biofilms are crucial to the pathogenesis of many important infections and are difficult to eradicate. Streptococcus suis is an important pathogen of pigs, and here the biofilm-forming ability of 32 strains of this species was determined. Significant biofilms were completely formed by 10 of the strains after 60 h of incubation, with exopolysaccharide production in the biofilm significantly higher than that in the corresponding planktonic cultures. S. suis strain SS2-4 formed a dense biofilm, as revealed by scanning electron microscopy, and in this state exhibited increased resistance to a number of antibiotics (ampicillin, amoxicillin, ciprofloxacin, kanamycin, and rifampin) compared to that of planktonic cultures. A bacteriophage lysin, designated LySMP, was used to attack biofilms alone and in combination with antibiotics and bacteriophage. The results demonstrated that the biofilms formed by S. suis, especially strains SS2-4 and SS2-H, could be dispersed by LySMP and with >80% removal compared to a biofilm reduction by treatment with either antibiotics or bacteriophage alone of less than 20%; in addition to disruption of the biofilm structure, the S. suis cells themselves were inactivated by LySMP. The efficacy of LySMP was not dose dependent, and in combination with antibiotics, it acted synergistically to maximize dispersal of the S. suis biofilm and inactivate the released cells. These data suggest that bacteriophage lysin could form part of an effective strategy to treat S. suis infections and represents a new class of antibiofilm agents.
Figures



Similar articles
-
Purified recombinant phage lysin LySMP: an extensive spectrum of lytic activity for swine streptococci.Curr Microbiol. 2009 Jun;58(6):609-15. doi: 10.1007/s00284-009-9379-x. Epub 2009 Mar 7. Curr Microbiol. 2009. PMID: 19267155
-
Csl2, a novel chimeric bacteriophage lysin to fight infections caused by Streptococcus suis, an emerging zoonotic pathogen.Sci Rep. 2017 Nov 28;7(1):16506. doi: 10.1038/s41598-017-16736-0. Sci Rep. 2017. PMID: 29184097 Free PMC article.
-
Fibrinogen induces biofilm formation by Streptococcus suis and enhances its antibiotic resistance.Appl Environ Microbiol. 2008 Aug;74(15):4969-72. doi: 10.1128/AEM.00558-08. Epub 2008 Jun 6. Appl Environ Microbiol. 2008. PMID: 18539785 Free PMC article.
-
Streptococcus suis biofilm: regulation, drug-resistance mechanisms, and disinfection strategies.Appl Microbiol Biotechnol. 2018 Nov;102(21):9121-9129. doi: 10.1007/s00253-018-9356-z. Epub 2018 Sep 12. Appl Microbiol Biotechnol. 2018. PMID: 30209548 Review.
-
Rethinking the control of Streptococcus suis infection: Biofilm formation.Vet Microbiol. 2024 Mar;290:110005. doi: 10.1016/j.vetmic.2024.110005. Epub 2024 Jan 21. Vet Microbiol. 2024. PMID: 38280304 Review.
Cited by
-
Aerococcus viridans Phage Lysin AVPL Had Lytic Activity against Streptococcus suis in a Mouse Bacteremia Model.Int J Mol Sci. 2023 Nov 23;24(23):16670. doi: 10.3390/ijms242316670. Int J Mol Sci. 2023. PMID: 38068990 Free PMC article.
-
Rapid-killing efficacy substantiates the antiseptic property of the synergistic combination of carvacrol and nerol against nosocomial pathogens.Arch Microbiol. 2022 Sep 2;204(9):590. doi: 10.1007/s00203-022-03197-x. Arch Microbiol. 2022. PMID: 36053368 Free PMC article.
-
Endolysins against Streptococci as an antibiotic alternative.Front Microbiol. 2022 Aug 2;13:935145. doi: 10.3389/fmicb.2022.935145. eCollection 2022. Front Microbiol. 2022. PMID: 35983327 Free PMC article. Review.
-
Effectiveness of Bacteriophages against Biofilm-Forming Shiga-Toxigenic Escherichia coli In Vitro and on Food-Contact Surfaces.Foods. 2023 Jul 22;12(14):2787. doi: 10.3390/foods12142787. Foods. 2023. PMID: 37509879 Free PMC article.
-
In Vitro Activity of the Bacteriophage Endolysin HY-133 against Staphylococcus aureus Small-Colony Variants and Their Corresponding Wild Types.Int J Mol Sci. 2019 Feb 7;20(3):716. doi: 10.3390/ijms20030716. Int J Mol Sci. 2019. PMID: 30736446 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

