The LRRC26 protein selectively alters the efficacy of BK channel activators
- PMID: 21984254
- PMCID: PMC3250112
- DOI: 10.1124/mol.111.075234
The LRRC26 protein selectively alters the efficacy of BK channel activators
Erratum in
- Mol Pharmacol. 2016 Jan;89(1):214
-
Correction to"The LRRC26 Protein Selectively Alters the Efficacy of BK Channel Activators".Mol Pharmacol. 2016 Jan;89(1):214. doi: 10.1124/mol.115.075234err. Mol Pharmacol. 2016. PMID: 31265510 Free PMC article.
Abstract
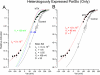
Large conductance, Ca(2+)-activated K channel proteins are involved in a wide range of physiological activities, so there is considerable interest in the pharmacology of large conductance calcium-activated K (BK) channels. One potent activator of BK channels is mallotoxin (MTX), which produces a very large hyperpolarizing shift of the voltage gating of heterologously expressed BK channels and causes a dramatic increase in the activity of BK channels in human smooth muscle cells. However, we found that MTX shifted the steady-state activation of BK channels in native parotid acinar cells by only 6 mV. This was not because the parotid BK isoform (parSlo) is inherently insensitive to MTX as MTX shifted the activation of heterologously expressed parSlo channels by 70 mV. Even though MTX had a minimal effect on steady-state activation of parotid BK channels, it produced an approximate 2-fold speeding of the channel-gating kinetics. The BK channels in parotid acinar cells have a much more hyperpolarized voltage activation range than BK channels in most other cell types. We found that this is probably attributable to an accessory protein, LRRC26, which is expressed in parotid glands: expressed parSlo + LRRC26 channels were resistant to the actions of MTX. Another class of BK activators is the benzimidazalones that includes 1,3-dihydro-1-(2-hydroxy-5-(trifluoromethyl)phenyl)-5-(trifluoromethyl)-2H-benzimidazol-2-one (NS-1619). Although the LRRC26 accessory protein strongly inhibited the ability of MTX to activate BK channels, we found that it had only a small effect on the action of NS-1619 on BK channels. Thus, the LRRC26 BK channel accessory protein selectively alters the pharmacology of BK channels.
Figures






References
-
- Brayden JE, Nelson MT. (1992) Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256:532–535 - PubMed
-
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. (1993) mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261:221–224 - PubMed
-
- Diaz L, Meera P, Amigo J, Stefani E, Alvarez O, Toro L, Latorre R. (1998) Role of the S4 segment in a voltage-dependent calcium-sensitive potassium (hSlo) channel. J Biol Chem 273:32430–32436 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

