PTPN2, a candidate gene for type 1 diabetes, modulates pancreatic β-cell apoptosis via regulation of the BH3-only protein Bim
- PMID: 21984578
- PMCID: PMC3219938
- DOI: 10.2337/db11-0758
PTPN2, a candidate gene for type 1 diabetes, modulates pancreatic β-cell apoptosis via regulation of the BH3-only protein Bim
Abstract
Objective: Genome-wide association studies allowed the identification of several associations between specific loci and type 1 diabetes (T1D). However, the mechanisms by which most candidate genes predispose to T1D remain unclear. We presently evaluated the mechanisms by which PTPN2, a candidate gene for T1D, modulates β-cell apoptosis after exposure to type I and II interferons (IFNs), cytokines that contribute to β-cell loss in early T1D.
Research design and methods: Small interfering RNAs were used to inhibit PTPN2, STAT1, Bim, and Jun NH(2)-terminal kinase 1 (JNK1) expression. Cell death was assessed by Hoechst and propidium iodide staining. BAX translocation, Bim phosphorylation, cytochrome c release, and caspases 9 and 3 activation were measured by Western blot or immunofluorescence.
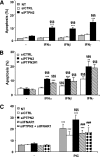
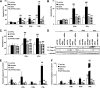
Results: PTPN2 knockdown exacerbated type I IFN-induced apoptosis in INS-1E, primary rat, and human β-cells. PTPN2 silencing and exposure to type I and II IFNs induced BAX translocation to the mitochondria, cytochrome c release, and caspase 3 activation. There was also an increase in Bim phosphorylation that was at least in part regulated by JNK1. Of note, both Bim and JNK1 knockdown protected β-cells against IFN-induced apoptosis in PTPN2-silenced cells.
Conclusions: The present findings suggest that local IFN production may interact with a genetic factor (PTPN2) to induce aberrant proapoptotic activity of the BH3-only protein Bim, resulting in increased β-cell apoptosis via JNK activation and the intrinsic apoptotic pathway. This is the first indication of a direct interaction between a candidate gene for T1D and the activation of a specific downstream proapoptotic pathway in β-cells.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

