Translocation and the alternative D-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the D-mannose/L-galactose pathway
- PMID: 21984649
- PMCID: PMC3245467
- DOI: 10.1093/jxb/err275
Translocation and the alternative D-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the D-mannose/L-galactose pathway
Abstract
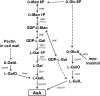
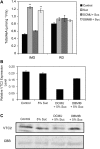
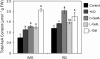
The D-mannose/L-galactose pathway for the biosynthesis of vitamin C (L-ascorbic acid; AsA) has greatly improved the understanding of this indispensable compound in plants, where it plays multifunctional roles. However, it is yet to be proven whether the same pathway holds for all the different organs of plants, especially the fruit-bearing plants, at different stages of development. Micro-Tom was used here to elucidate the mechanisms of AsA accumulation and regulation in tomato fruits. The mRNA expression of the genes in the D-mannose/L-galactose pathway were inversely correlated with increasing AsA content of Micro-Tom fruits during ripening. Feeding L-[6-(14)C]AsA to Micro-Tom plants revealed that the bulk of the label from AsA accumulated in the source leaf was transported to the immature green fruits, and the rate of translocation decreased as ripening progressed. L-Galactose feeding, but neither D-galacturonate nor L-gulono-1,4-lactone, enhanced the content of AsA in immature green fruit. On the other hand, L-galactose and D-galacturonate, but not L-gulono-1,4-lactone, resulted in an increase in the AsA content of red ripened fruits. Crude extract prepared from insoluble fractions of green and red fruits showed D-galacturonate reductase- and aldonolactonase-specific activities, the antepenultimate and penultimate enzymes, respectively, in the D-galacturonate pathway, in both fruits. Taken together, the present findings demonstrated that tomato fruits could switch between different sources for AsA supply depending on their ripening stages. The translocation from source leaves and biosynthesis via the D-mannose/L-galactose pathway are dominant sources in immature fruits, while the alternative D-galacturonate pathway contributes to AsA accumulation in ripened Micro-Tom fruits.
Figures


References
-
- Agius F, Gonzalez-Lamonthe R, Caballero JL, Munoz-Blanco J, Botella MA, Valpuesta V. Engineering increased vitamin C levels in plants by over-expression of a D-galacturonic acid reductase. Nature Biotechnology. 2003;21:177–181. - PubMed
-
- Badejo AA, Fujikawa Y, Esaka M. Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff–Wheeler pathway in acerola (Malpighia glabra) Journal of Plant Physiology. 2009;166:652–660. - PubMed
-
- Bulley SM, Rassam M, Hoser D, Otto W, Schunemann N, Wright M, MacRae E, Gleave A, Laing W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. Journal of Experimental Botany. 2009;60:765–778. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

