Regulation of the competence pathway as a novel role associated with a streptococcal bacteriocin
- PMID: 21984782
- PMCID: PMC3232909
- DOI: 10.1128/JB.05968-11
Regulation of the competence pathway as a novel role associated with a streptococcal bacteriocin
Abstract
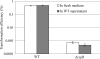
The oral biofilm organism Streptococcus mutans must face numerous environmental stresses to survive in its natural habitat. Under specific stresses, S. mutans expresses the competence-stimulating peptide (CSP) pheromone known to induce autolysis and facilitate the uptake and incorporation of exogenous DNA, a process called DNA transformation. We have previously demonstrated that the CSP-induced CipB bacteriocin (mutacin V) is a major factor involved in both cellular processes. Our objective in this work was to characterize the role of CipB bacteriocin during DNA transformation. Although other bacteriocin mutants were impaired in their ability to acquire DNA under CSP-induced conditions, the ΔcipB mutant was the only mutant showing a sharp decrease in transformation efficiency. The autolysis function of CipB bacteriocin does not participate in the DNA transformation process, as factors released via lysis of a subpopulation of cells did not contribute to the development of genetic competence in the surviving population. Moreover, CipB does not seem to participate in membrane depolarization to assist passage of DNA. Microarray-based expression profiling showed that under CSP-induced conditions, CipB regulated ∼130 genes, among which are the comDE locus and comR and comX genes, encoding critical factors that influence competency development in S. mutans. We also discovered that the CipI protein conferring immunity to CipB-induced autolysis also prevented the transcriptional regulatory activity of CipB. Our data suggest that besides its role in cell lysis, the S. mutans CipB bacteriocin also functions as a peptide regulator for the transcriptional control of the competence regulon.
Figures

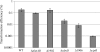

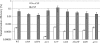

References
-
- Abee T. 1995. Pore-forming bacteriocins of Gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol. Lett. 129:1–10 - PubMed
-
- Alix E., Blanc-Potard A. B. 2009. Hydrophobic peptides: novel regulators within bacterial membrane. Mol. Microbiol. 72:5–11 - PubMed
-
- Balakrishnan M., Simmonds R. S., Kilian M., Tagg J. R. 2002. Different bacteriocin activities of Streptococcus mutans reflect phylogenetic lineages. J. Med. Microbiol. 51:941–948 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

