Activation of anti-tumor immune response and reduction of regulatory T cells with Mycobacterium indicus pranii (MIP) therapy in tumor bearing mice
- PMID: 21984926
- PMCID: PMC3184142
- DOI: 10.1371/journal.pone.0025424
Activation of anti-tumor immune response and reduction of regulatory T cells with Mycobacterium indicus pranii (MIP) therapy in tumor bearing mice
Abstract
Background: Role of immune system in protecting the host from cancer is well established. Growing cancer however subverts immune response towards Th2 type and escape from antitumor mechanism of the host. Activation of both innate and Th1 type response is crucial for host antitumor activity. In our previous study it was found, that Mycobacterium indicus pranii (MIP) also known as M. w induces Th1 type response and activates macrophages in animal model of tuberculosis. Hence, we studied the immunotherapeutic potential of MIP in mouse tumor model and the underlying mechanisms for its antitumor activity.
Methodology and principal findings: Tumors were implanted by injecting B16F10 melanoma cells subcutaneously into C57BL/6 mice. Using the optimized dose and treatment regimes, anti-tumor efficacy of heat killed MIP was evaluated. In MIP treated group, tumor appeared in only 50-60% of mice, tumor growth was delayed and tumor volume was less as compared to control. MIP mediated immune activation was analysed in the tumor microenvironment, tumor draining lymph node and spleen. Induction of Th1 response and higher infiltration of immune cells in the tumor microenvironment was observed in MIP treated mice. A large fraction of these immune cells were in activated state as confirmed by phenotypic and functional analysis. Interestingly, percentage of Treg cells in the tumor milieu of treated mice was less. We also evaluated efficacy of MIP along with chemotherapy and found a better response as compared to chemotherapy alone.
Conclusion: MIP therapy is effective in protecting mice from tumor. It activates the immune cells, increases their infiltration in tumor, and abrogates tumor mediated immune suppression.
Conflict of interest statement
Figures
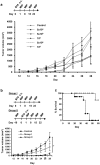

 ) unlabelled cells; (––) labelled cells.
) unlabelled cells; (––) labelled cells.
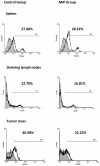
 ) CD4+ cells; (––) CD4+FoxP3+ cells.
) CD4+ cells; (––) CD4+FoxP3+ cells.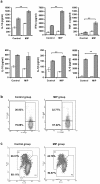

References
-
- Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. - PubMed
-
- Park SH, Kyin T, Bendelac A, Carnaud C. The contribution of NKT cells, NK cells, and other gamma-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J Immunol. 2003;170:1197–1201. - PubMed
-
- Baxevanis CN, Gritzapis AD, Papamichail M. In vivo antitumor activity of NKT cells activated by the combination of IL-12 and IL-18. J Immunol. 2003;171:2953–2959. - PubMed
-
- Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–1694. - PubMed
-
- Hayashi A, Doi O, Azuma I, Toyoshima K. Immuno-friendly use of BCG-cell wall skeleton remarkably improves the survival rate of various cancer patients. Proc Japn Acad. 1998;74:50–55.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

