A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology
- PMID: 21984937
- PMCID: PMC3184152
- DOI: 10.1371/journal.pone.0025661
A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology
Abstract
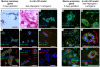
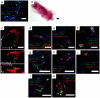
Studies on the stem cell niche and the efficacy of cancer therapeutics require complex multicellular structures and interactions between different cell types and extracellular matrix (ECM) in three dimensional (3D) space. We have engineered a 3D in vitro model of mammary gland that encompasses a defined, porous collagen/hyaluronic acid (HA) scaffold forming a physiologically relevant foundation for epithelial and adipocyte co-culture. Polarized ductal and acinar structures form within this scaffold recapitulating normal tissue morphology in the absence of reconstituted basement membrane (rBM) hydrogel. Furthermore, organoid developmental outcome can be controlled by the ratio of collagen to HA, with a higher HA concentration favouring acinar morphological development. Importantly, this culture system recapitulates the stem cell niche as primary mammary stem cells form complex organoids, emphasising the utility of this approach for developmental and tumorigenic studies using genetically altered animals or human biopsy material, and for screening cancer therapeutics for personalised medicine.
Conflict of interest statement
Figures


References
-
- Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135:995–1003. - PubMed
-
- Dado D, Levenberg S. Cell–scaffold mechanical interplay within engineered tissue. Seminars in Cell and Developmental Biology. 2009;20:656–664. - PubMed
-
- Eheman CR, Shaw KM, Ryerson AB, Miller JW, Ajani UA, et al. The changing incidence of in situ and invasive ductal and lobular breast carcinomas: United States, 1999-2004. Cancer Epidemiol Biomarkers Prev. 2009;18:1763–1769. - PubMed
-
- Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the α2β1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation. 2005;59:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

