Catecholamines up integrates dopamine synthesis and synaptic trafficking
- PMID: 21985068
- PMCID: PMC3233821
- DOI: 10.1111/j.1471-4159.2011.07517.x
Catecholamines up integrates dopamine synthesis and synaptic trafficking
Abstract
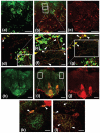
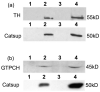
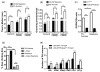
The highly reactive nature of dopamine renders dopaminergic neurons vulnerable to oxidative damage. We recently demonstrated that loss-of-function mutations in the Drosophila gene Catecholamines up (Catsup) elevate dopamine pools but, paradoxically, also confer resistance to paraquat, an herbicide that induces oxidative stress-mediated toxicity in dopaminergic neurons. We now report a novel association of the membrane protein, Catsup, with GTP cyclohydrolase rate-limiting enzyme for tetrahydrobiopterin (BH(4)) biosynthesis and tyrosine hydroxylase, rate-limiting enzyme for dopamine biosynthesis, which requires BH(4) as a cofactor. Loss-of-function Catsup mutations cause dominant hyperactivation of both enzymes. Elevated dopamine levels in Catsup mutants coincide with several distinct characteristics, including hypermobility, minimal basal levels of 3,4-dihydroxy-phenylacetic acid, an oxidative metabolite of dopamine, and resistance to the vesicular monoamine transporter inhibitor, reserpine, suggesting that excess dopamine is synaptically active and that Catsup functions in the regulation of synaptic vesicle loading and release of dopamine. We conclude that Catsup regulates and links the dopamine synthesis and transport networks.
© 2011 The Authors. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Figures


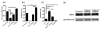

References
-
- Asanuma M, Miyazaki I, Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res. 2003;5:165–76. - PubMed
-
- Bayersdorfer F, Voigt A, Schneuwly S, Botella JA. Dopamine-dependent neurodegeneration in Drosophila models of familial and sporadic Parkinson’s disease. Neurobiol Dis. 2010 In Press. - PubMed
-
- Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and Induces permeability transition in brain mitochondria: Implications for Parkinson’s disease. J Neurochem. 1999;73:1127–1137. - PubMed
-
- Bonini NM, Fortini ME. Human neurodegenerative disease modeling using Drosophila. Annu. Rev. Neurosci. 2003;26:627–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

