Suppressive role of hepatic dendritic cells in concanavalin A-induced hepatitis
- PMID: 21985372
- PMCID: PMC3219901
- DOI: 10.1111/j.1365-2249.2011.04458.x
Suppressive role of hepatic dendritic cells in concanavalin A-induced hepatitis
Abstract
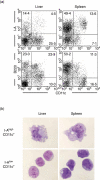
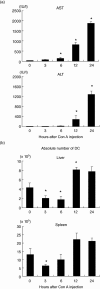
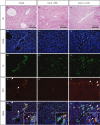
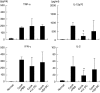
Concanavalin A (Con A)-induced hepatitis is a mouse model of acute autoimmune hepatitis. The aim of this study was to investigate the role of hepatic dendritic cells (DC) in the immune modulation of tissue damage. Almost all hepatic DC were plasmacytoid DC (CD11c+ I-A(low) B220+); however, conventional DC were CD11c+ I-A(high) B220(-). At an early stage (3-6 h) after Con A administration, the number of DC in both the liver and spleen decreased, increasing thereafter (12-24 h) in parallel with hepatic failure. The hepatic CD11c+ DC population contained many CD11b(-) cells, while the majority of splenic CD11c+ DC were CD11b+. After Con A administration, the proportion of I-A+ and CD11b+ cells within the CD11c+ DC population tended to increase in the liver, but not in the spleen. Similarly, expression of the activation markers CD80, CD86 and CD40 by CD11c+ DC increased in the liver, but not in the spleen. Next, adoptive transfer of DC isolated from the liver and spleen was performed 3 h after Con A administration to examine the immunomodulatory function of DC. Only hepatic DC had the ability to suppress hepatic failure. Analysis of cytokine production and subsequent identification of the effector cells showed that hepatic DC achieved this by suppressing the production of interleukin (IL)-12 and IL-2, rather than modulating effector cell function.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures





References
-
- Jomantaite I, Dikopoulos N, Kröger A, et al. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–65. - PubMed
-
- Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445–54. - PubMed
-
- Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

