Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease
- PMID: 21985374
- PMCID: PMC3219903
- DOI: 10.1111/j.1365-2249.2011.04471.x
Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease
Abstract
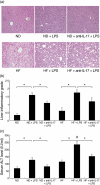
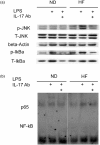
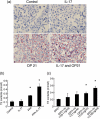
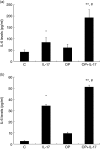
Mechanisms associated with the progression of simple steatosis to non-alcoholic fatty liver disease (NAFLD) remain undefined. Regulatory T cells (T(regs)) play a critical role in regulating inflammatory processes in non-alcoholic steatohepatitis (NASH) and because T helper type 17 (Th17) functionally oppose T(reg)-mediated responses, this study focused on characterizing the role of Th17 cells using a NAFLD mouse model. C57BL/6 mice were fed either a normal diet (ND) or high fat (HF) diet for 8 weeks. Mice in the HF group had a significantly higher frequency of liver Th17 cells compared to ND-fed mice. Neutralization of interleukin (IL)-17 in HF mice ameliorated lipopolysaccharide (LPS)-induced liver injury reflected by decreased serum alanine aminotransferase (ALT) levels and reduced inflammatory cell infiltrates in the liver. In vitro, HepG2 cells cultured in the presence of free fatty acids (FFA; oleic acid and palmitic acid) for 24 h and IL-17 developed steatosis via insulin-signalling pathway interference. IL-17 and FFAs synergized to induce IL-6 production by HepG2 cells and murine primary hepatocytes which, in combination with transforming growth factor (TGF-β), expanded Th17 cells. It is likely that a similar process occurs in NASH patients, as there were significant levels of IL-17(+) cell infiltrates in NASH patient livers. The hepatic expression of Th17 cell-related genes [retinoid-related orphan receptor gamma (ROR)γt, IL-17, IL-21 and IL-23] was also increased significantly in NASH patients compared to healthy controls. Th17 cells and IL-17 were associated with hepatic steatosis and proinflammatory response in NAFLD and facilitated the transition from simple steatosis to steatohepatitis. Strategies designed to alter the balance between Th17 cells and T(regs) should be explored as a means of preventing progression to NASH and advanced liver diseases in NAFLD patients.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures



References
-
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(Suppl. 1):S99–S112. - PubMed
-
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. - PubMed
-
- Brunt EM. Nonalcoholic steatohepatitis. Semin Liver Dis. 2004;24:3–20. - PubMed
-
- Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197–208. - PubMed
-
- Kremer M, Hines IN, Milton RJ, Wheeler MD. Favored T helper 1 response in a mouse model of hepatosteatosis is associated with enhanced T cell-mediated hepatitis. Hepatology. 2006;44:216–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

