The time since last menstrual period is important as a clinical predictor for non-steroidal aromatase inhibitor-related arthralgia
- PMID: 21985669
- PMCID: PMC3198721
- DOI: 10.1186/1471-2407-11-436
The time since last menstrual period is important as a clinical predictor for non-steroidal aromatase inhibitor-related arthralgia
Abstract
Background: The clinical predictors of aromatase inhibitor-related arthralgia (AIA), a drug-related adverse reaction of aromatase inhibitors (AIs), remain unclear.
Methods: AIA was prospectively surveyed every 4 months in 328 postmenopausal breast cancer patients administered a non-steroidal AI (anastrozole). Various clinicopathological parameters were recorded and analyzed (chi-square test, Fisher's exact test and logistic regression analysis).
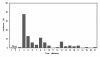
Results: The mean observation period was 39.9 months. AIA manifested in 114 patients (34.8%), with peaks of onset at 4 (33.7%) and 8 months (11.4%) after starting AI administration. Some cases manifested even after 13 months. AIA tended to occur in younger patients (incidences of 46.3%, 37.4% and 28.0% for ages of < 55, 55-65 and > 65 years, respectively (p = 0.063)) and decreased significantly with the age at menarche (53.3%, 35.3% and 15.4% for < 12, 12-15 and > 15 years, respectively (p = 0.036)). The incidences were 45.1%, 46.3 and 25.1% for the time since the last menstrual period (LMP) < 5 years, 5-10 years and > 10 years, being significantly lower at > 10 years (p < 0.001). In logistic regression analysis, the AIA incidence was significantly lower in the time since LMP > 10-year group versus the < 5-year group (odds ratio 0.44, p = 0.002), but the age at menarche showed no association. AIA manifested significantly earlier (≤ 6 months) as the time since LMP became shorter (< 5 years).
Conclusion: AIA tends to manifest early after starting AI, but some cases show delayed onset. The incidence was significantly lower in patients with a duration of > 10 years since LMP. When the time since LMP was short, the onset of AIA was significantly earlier after starting AI administration.
Figures
Similar articles
-
Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors.Cancer. 2009 Aug 15;115(16):3631-9. doi: 10.1002/cncr.24419. Cancer. 2009. PMID: 19517460 Free PMC article.
-
Adjuvant aromatase inhibitor therapy in early breast cancer: what factors lead patients to discontinue treatment?Tumori. 2015 Sep-Oct;101(5):469-73. doi: 10.5301/tj.5000376. Epub 2015 Jun 13. Tumori. 2015. PMID: 26108239
-
Aromatase inhibitor-associated arthralgias: Pathogenesis, frequency and management.Joint Bone Spine. 2007 Dec;74(6):662-3. doi: 10.1016/j.jbspin.2007.04.010. Epub 2007 Aug 13. Joint Bone Spine. 2007. PMID: 17913550 No abstract available.
-
[Aromatase inhibitors and arthralgia].Magy Onkol. 2011 Mar;55(1):32-9. Epub 2011 Mar 31. Magy Onkol. 2011. PMID: 21617789 Review. Hungarian.
-
Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: A meta-analysis of randomized controlled trials.Breast. 2017 Jun;33:132-138. doi: 10.1016/j.breast.2017.03.015. Epub 2017 Apr 4. Breast. 2017. PMID: 28384564 Review.
Cited by
-
Current and future advances in practice: aromatase inhibitor-induced arthralgia.Rheumatol Adv Pract. 2024 Apr 10;8(2):rkae024. doi: 10.1093/rap/rkae024. eCollection 2024. Rheumatol Adv Pract. 2024. PMID: 38601139 Free PMC article. Review.
-
Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer.Cochrane Database Syst Rev. 2022 Jan 10;1(1):CD013167. doi: 10.1002/14651858.CD013167.pub2. Cochrane Database Syst Rev. 2022. PMID: 35005781 Free PMC article.
-
Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic review.PLoS One. 2014 May 9;9(5):e96748. doi: 10.1371/journal.pone.0096748. eCollection 2014. PLoS One. 2014. PMID: 24816774 Free PMC article.
-
Germline genetic predictors of aromatase inhibitor concentrations, estrogen suppression and drug efficacy and toxicity in breast cancer patients.Pharmacogenomics. 2017 Apr;18(5):481-499. doi: 10.2217/pgs-2016-0205. Epub 2017 Mar 27. Pharmacogenomics. 2017. PMID: 28346074 Free PMC article. Review.
-
Aerobic exercise and aromatase inhibitor-associated musculoskeletal symptoms: results of a randomized clinical trial.Support Care Cancer. 2025 Mar 4;33(3):244. doi: 10.1007/s00520-025-09257-4. Support Care Cancer. 2025. PMID: 40035875 Clinical Trial.
References
-
- Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Brynt J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. - PubMed
-
- Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology. 2008;22:1401–1408. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical