Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury
- PMID: 21985786
- PMCID: PMC3204843
- DOI: 10.1172/JCI58097
Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury
Abstract
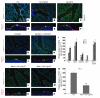
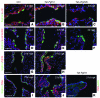
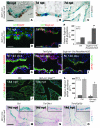
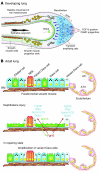
During lung development, parabronchial SMC (PSMC) progenitors in the distal mesenchyme secrete fibroblast growth factor 10 (Fgf10), which acts on distal epithelial progenitors to promote their proliferation. β-catenin signaling within PSMC progenitors is essential for their maintenance, proliferation, and expression of Fgf10. Here, we report that this Wnt/Fgf10 embryonic signaling cascade is reactivated in mature PSMCs after naphthalene-induced injury to airway epithelium. Furthermore, we found that this paracrine Fgf10 action was essential for activating surviving variant Clara cells (the cells in the airway epithelium from which replacement epithelial cells originate) located at the bronchoalveolar duct junctions and adjacent to neuroendocrine bodies. After naphthalene injury, PSMCs secreted Fgf10 to activate Notch signaling and induce Snai1 expression in surviving variant Clara cells, which subsequently underwent a transient epithelial to mesenchymal transition to initiate the repair process. Epithelial Snai1 expression was important for regeneration after injury. We have therefore identified PSMCs as a stem cell niche for the variant Clara cells in the lung and established that paracrine Fgf10 signaling from the niche is critical for epithelial repair after naphthalene injury. These findings also have implications for understanding the misregulation of lung repair in asthma and cancer.
Figures


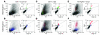

References
-
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124(23):4867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

