Prognostic and predictive role of ESR1 status for postmenopausal patients with endocrine-responsive early breast cancer in the Danish cohort of the BIG 1-98 trial
- PMID: 21986093
- PMCID: PMC3335246
- DOI: 10.1093/annonc/mdr438
Prognostic and predictive role of ESR1 status for postmenopausal patients with endocrine-responsive early breast cancer in the Danish cohort of the BIG 1-98 trial
Abstract
Background: Estrogen Receptor 1 (ESR1) aberrations may be associated with expression of estrogen receptor (ER) or progesterone receptor (PgR), human epidermal growth factor receptor-2 (HER2) or Ki-67 labeling index and prognosis.
Patients and methods: ESR1 was assessed in 1129 (81%) of 1396 postmenopausal Danish women with early breast cancer randomly assigned to receive 5 years of letrozole, tamoxifen or a sequence of these agents in the Breast International Group 1-98 trial and who had ER ≥ 1% after central review.
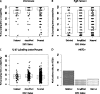
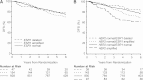
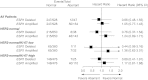
Results: By FISH, 13.6% of patients had an ESR1-to-Centromere-6 (CEN-6) ratio ≥ 2 (amplified), and 4.2% had ESR1-to-CEN-6 ratio <0.8 (deleted). Deletion of ESR1 was associated with significantly lower levels of ER (P < 0.0001) and PgR (P = 0.02) and more frequent HER2 amplification. ESR1 deletion or amplification was associated with higher-Ki-67 than ESR1-normal tumors. Overall, there was no evidence of heterogeneity of disease-free survival (DFS) or in treatment effect according to ESR1 status. However, significant differences in DFS were observed for subsets based on a combination of ESR1 and HER2 status (P = 0.02).
Conclusions: ESR1 aberrations were associated with HER2 status, Ki-67 labeling index and ER and PgR levels. When combined with HER2, ESR1 may be prognostic but should not be used for endocrine treatment selection in postmenopausal women with endocrine-responsive early breast cancer.
Figures
References
-
- Early Breast Cancer Trialists Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. - PubMed
-
- Sotiriou C, Piccart MJ. Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat Rev Cancer. 2007;7(7):545–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

