The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease
- PMID: 21986389
- PMCID: PMC3253961
- DOI: 10.1016/j.cct.2011.09.009
The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease
Abstract
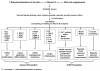
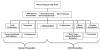
Data from laboratory studies, observational research, and/or secondary prevention trials suggest that vitamin D and marine omega-3 fatty acids may reduce risk for cancer or cardiovascular disease (CVD), but primary prevention trials with adequate dosing in general populations (i.e., unselected for disease risk) are lacking. The ongoing VITamin D and OmegA-3 TriaL (VITAL) is a large randomized, double-blind, placebo-controlled, 2 x 2 factorial trial of vitamin D (in the form of vitamin D(3) [cholecalciferol], 2000 IU/day) and marine omega-3 fatty acid (Omacor fish oil, eicosapentaenoic acid [EPA]+docosahexaenoic acid [DHA], 1g/day) supplements in the primary prevention of cancer and CVD among a multi-ethnic population of 20,000 U.S. men aged ≥ 50 and women aged ≥ 55. The mean treatment period will be 5 years. Baseline blood samples will be collected in at least 16,000 participants, with follow-up blood collection in about 6000 participants. Yearly follow-up questionnaires will assess treatment compliance (plasma biomarker measures will also assess compliance in a random sample of participants), use of non-study drugs or supplements, occurrence of endpoints, and cancer and vascular risk factors. Self-reported endpoints will be confirmed by medical record review by physicians blinded to treatment assignment, and deaths will be ascertained through national registries and other sources. Ancillary studies will investigate whether these agents affect risk for diabetes and glucose intolerance; hypertension; cognitive decline; depression; osteoporosis and fracture; physical disability and falls; asthma and other respiratory diseases; infections; and rheumatoid arthritis, systemic lupus erythematosus, thyroid diseases, and other autoimmune disorders.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–36. - PubMed
-
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. - PubMed
-
- Manson JE, Bassuk SS. Vitamin D and cardiovascular disease. Menopause Management. 2009;18:28–31.
-
- Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–75. - PubMed
-
- Grant WB. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003;164:371–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK088762/DK/NIDDK NIH HHS/United States
- R01AR059086/AR/NIAMS NIH HHS/United States
- R01 HL102122/HL/NHLBI NIH HHS/United States
- R01 DK088078/DK/NIDDK NIH HHS/United States
- R01 HL101932/HL/NHLBI NIH HHS/United States
- R01 AR059775/AR/NIAMS NIH HHS/United States
- U01 CA138962/CA/NCI NIH HHS/United States
- R01 AR059086/AR/NIAMS NIH HHS/United States
- R01 AG036755/AG/NIA NIH HHS/United States
- R01 MH091448/MH/NIMH NIH HHS/United States
- R01 AR060574/AR/NIAMS NIH HHS/United States
- R01AR060574/AR/NIAMS NIH HHS/United States
- R01MH091448/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

