Surface lipoprotein PpiA of Streptococcus mutans suppresses scavenger receptor MARCO-dependent phagocytosis by macrophages
- PMID: 21986627
- PMCID: PMC3232644
- DOI: 10.1128/IAI.05693-11
Surface lipoprotein PpiA of Streptococcus mutans suppresses scavenger receptor MARCO-dependent phagocytosis by macrophages
Abstract
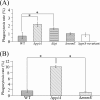
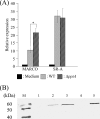
Streptococcus mutans is associated with the initiation and progression of human dental caries and is occasionally isolated from the blood of patients with bacteremia and infective endocarditis. For the pathogen to survive in the infected host, surface lipoproteins of S. mutans are likely to play important roles in interactions with the innate immune system. To clarify the role that a putative lipoprotein, peptidyl-prolyl cis/trans-isomerase (PpiA), of S. mutans plays in the macrophage response, we investigated the response of THP-1-derived macrophages to S. mutans challenge. The deletion of the gene encoding Lgt eliminated PpiA on the cell surface of S. mutans, which implies that PpiA is a lipoprotein that is lipid anchored in the cell membrane by Lgt. Human and murine peritoneal macrophages both showed higher phagocytic activities for the ppiA and lgt mutants than the wild type, which indicates that the presence of PpiA reduces S. mutans phagocytosis. In addition, infection with S. mutans markedly induced mRNAs of macrophage receptor with collagenous structure (MARCO) and scavenger receptor A (SR-A) in human macrophages. In particular, transcriptional and translational levels of MARCO in human macrophages infected with the ppiA mutant were higher than those in macrophages infected with the wild type. Phagocytosis of S. mutans by human macrophages markedly decreased after treatment with anti-MARCO IgG. These results demonstrate that the S. mutans lipoprotein PpiA contributes to suppression of MARCO-mediated phagocytosis of this bacterium by macrophages.
Figures



References
-
- Akira S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5–11 - PubMed
-
- Alloing G., Phillip F., Claverys J. P. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of Gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44–58 - PubMed
-
- Areschoug T., Gordon S. 2008. Pattern recognition receptors and their role in innate immunity: focus on microbial protein ligands. Contrib. Microbiol. 15:45–60 - PubMed
-
- Areschoug T., Waldemarsson J., Gordon S. 2008. Evasion of macrophage scavenger receptor A-mediated recognition by pathogenic streptococci. Eur. J. Immunol. 38:3068–3079 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

