Interplay between the gastric bacterial microbiota and Candida albicans during postantibiotic recolonization and gastritis
- PMID: 21986629
- PMCID: PMC3255670
- DOI: 10.1128/IAI.05162-11
Interplay between the gastric bacterial microbiota and Candida albicans during postantibiotic recolonization and gastritis
Abstract
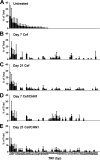
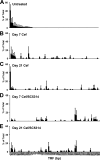
The indigenous bacterial microbiome of the stomach, including lactobacilli, is vital in promoting colonization resistance against Candida albicans. However, there are gaps in our understanding about C. albicans gastric colonization versus disease, especially during the postantibiotic recovery phase. This study compared the gastric responses to C. albicans strains CHN1 and SC5314 in microbiome-disturbed and germfree mice to elucidate the contribution of the indigenous microbiota in C. albicans colonization versus disease and yeast-bacterium antagonism during the post-cefoperazone recolonization period. C. albicans can prevent the regrowth of Lactobacillus spp. in the stomach after cefoperazone and promote increased colonization by Enterococcus spp. Using a culture-independent analysis, the effects of oral cefoperazone on the gastric bacterial microbiota were observed to last at least 3 weeks after the cessation of the antibiotic. Disturbance of the gastric bacterial community by cefoperazone alone was not sufficient to cause gastritis, C. albicans colonization was also needed. Gastritis was not evident until after day 7 in cefoperazone-treated infected mice. In contrast, in germfree mice which lack a gastric microbiota, C. albicans induced gastric inflammation within 1 week of inoculation. Therefore, the gastric bacterial community in cefoperazone-treated mice during the first week of postantibiotic recolonization was sufficient to prevent the development of gastritis, despite being ineffective at conferring colonization resistance against C. albicans. Altogether, these data implicate a dichotomy between C. albicans colonization and gastric disease that is bacterial microbiome dependent.
Figures



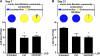
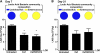

References
-
- Balish E, Jensen J, Warner T, Brekke J, Leonard B. 1993. Mucosal and disseminated candidiasis in gnotobiotic SCID mice. J. Med. Vet. Mycol. 31: 143–154 - PubMed
-
- Bistoni F, et al. 1993. Mucosal and systemic T helper cell function after intragastric colonization of adult mice with Candida albicans. J. Infect. Dis. 168: 1449–1457 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21-AI083473/AI/NIAID NIH HHS/United States
- KO8 DK0669907-01/DK/NIDDK NIH HHS/United States
- R01 DK070875/DK/NIDDK NIH HHS/United States
- R21-AI087869/AI/NIAID NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- R21 AI087869/AI/NIAID NIH HHS/United States
- R21 AI083473/AI/NIAID NIH HHS/United States
- R01 DK087708/DK/NIDDK NIH HHS/United States
- P30-DK034933/DK/NIDDK NIH HHS/United States
- R01 AI064479/AI/NIAID NIH HHS/United States
- R01-AI064479/AI/NIAID NIH HHS/United States
- R01-DK087708/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

