Population-related variation in plant defense more strongly affects survival of an herbivore than its solitary parasitoid wasp
- PMID: 21987026
- PMCID: PMC3197929
- DOI: 10.1007/s10886-011-0024-3
Population-related variation in plant defense more strongly affects survival of an herbivore than its solitary parasitoid wasp
Abstract
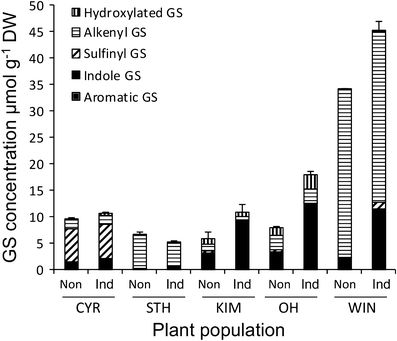
The performance of natural enemies, such as parasitoid wasps, is affected by differences in the quality of the host's diet, frequently mediated by species or population-related differences in plant allelochemistry. Here, we compared survival, development time, and body mass in a generalist herbivore, the cabbage moth, Mamestra brassicae, and its solitary endoparasitoid, Microplitis mediator, when reared on two cultivated (CYR and STH) and three wild (KIM, OH, and WIN) populations of cabbage, Brassica oleracea. Plants either were undamaged or induced by feeding of larvae of the cabbage butterfly, Pieris rapae. Development and biomass of M. brassicae and Mi. mediator were similar on both cultivated and one wild cabbage population (KIM), intermediate on the OH population, and significantly lower on the WIN population. Moreover, development was prolonged and biomass was reduced on herbivore-induced plants. However, only the survival of parasitized hosts (and not that of healthy larvae) was affected by induction. Analysis of glucosinolates in leaves of the cabbages revealed higher levels in the wild populations than cultivars, with the highest concentrations in WIN plants. Multivariate statistics revealed a negative correlation between insect performance and total levels of glucosinolates (GS) and levels of 3-butenyl GS. However, GS chemistry could not explain the reduced performance on induced plants since only indole GS concentrations increased in response to herbivory, which did not affect insect performance based on multivariate statistics. This result suggests that, in addition to aliphatic GS, other non-GS chemicals are responsible for the decline in insect performance, and that these chemicals affect the parasitoid more strongly than the host. Remarkably, when developing on WIN plants, the survival of Mi. mediator to adult eclosion was much higher than in its host, M. brassicae. This may be due to the fact that hosts parasitized by Mi. mediator pass through fewer instars, and host growth is arrested when they are only a fraction of the size of healthy caterpillars. Certain aspects of the biology and life-history of the host and parasitoid may determine their response to chemical challenges imposed by the food plant.
Figures



References
-
- Askew RR, Shaw MR. Parasitoid communities: their size, structure and development. In: Waage J, Greathead D, editors. Insect Parasitoids. London, UK: Academic; 1986. pp. 225–264.
-
- Barbosa P, Saunders JA, Kemper J, Trumbule R, Olechno J, Martinat P. Plant allelochemicals and insect parasitoids effects of nicotine on Cotesia congregata (Say) (Hymenoptera: Braconidae) and Hyposoter annulipes (Cresson) (Hymenoptera: Ichneumonidae) J. Chem. Ecol. 1986;12:1319–1328. doi: 10.1007/BF01012351. - DOI - PubMed
-
- Benrey B, Dennor RF. The slow growth-high mortality hypothesis: A test using the cabbage butterfly. Ecology. 1997;78:987–999.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

