Generation of mature monocyte-derived dendritic cells in the presence of heparin and monocyte conditioned medium: phenotypic and functional comparison
- PMID: 21987113
- PMCID: PMC3639743
Generation of mature monocyte-derived dendritic cells in the presence of heparin and monocyte conditioned medium: phenotypic and functional comparison
Abstract
Background: Dendritic cells (DC) induce tumor or pathogen-specific T cell responses in humans. Several laboratories have developed culture systems, including maturation factors for human DC from peripheral blood monocytes. We comprehensively compared standard maturation stimulus, an autologous monocyte-conditioned medium (MCM), with heparin for their ability to promote uniformly mature DC that elicit T cell responses.
Methods: A short (4-day) priming of plastic adherent monocytes with granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-4 with or without heparin was followed by 48-hour incubation in MCM to generate fully mature and stable DC. Phenotypic and functional analyses were carried out using anti-CD14 and anti-CD83 monoclonal antibodies, and mixed lymphocyte reaction, respectively.
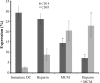
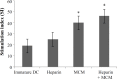
Results: We found that fully matured DC with a large amount of cytoplasm and copious dendritic projections were visible at the end of culturing period in the presence of MCM, heparin and MCM plus heparin. Thus, DC generated with these maturation factors are non-adherent and have typical satellite morphology. Flow cytometric analysis using anti-CD14 (monocyte marker) and anti-CD83 (mature DC marker) revealed that expression of CD14 decreased in MCM plus heparin-treated DC, and the expression of CD83 was increased when heparin and MCM used as a maturation factor. Functionally, MCM and MCM plus heparin-treated DC showed stronger mixed leukocyte reaction than heparin alone.
Conclusion: These results support the use of the MCM with heparin as maturation factor that could result in functionally mature monocyte-derived DC in comparison to either MCM or heparin alone.
Keywords: Dendritic cell; Maturation; Monocyte Conditioned Medium; Heparin.
Figures


Similar articles
-
Generation of dendritic cells from adherent cells of cord blood by culture with granulocyte-macrophage colony-stimulating factor, interleukin-4, and tumor necrosis factor-alpha.J Hematother Stem Cell Res. 2000 Aug;9(4):453-64. doi: 10.1089/152581600419116. J Hematother Stem Cell Res. 2000. PMID: 10982243
-
Mixture of fibroblast, epithelial and endothelial cells conditioned media induce monocyte-derived dendritic cell maturation.Cell Immunol. 2011;272(1):18-24. doi: 10.1016/j.cellimm.2011.10.001. Epub 2011 Oct 10. Cell Immunol. 2011. PMID: 22035776
-
Dendritic cells as the terminal stage of monocyte differentiation.J Immunol. 1998 May 1;160(9):4587-95. J Immunol. 1998. PMID: 9574566
-
Cancer Vaccines in the World of Immune Suppressive Monocytes (CD14(+)HLA-DR(lo/neg) Cells): The Gateway to Improved Responses.Front Immunol. 2014 Apr 4;5:147. doi: 10.3389/fimmu.2014.00147. eCollection 2014. Front Immunol. 2014. PMID: 24772111 Free PMC article. Review.
-
Dendritic Cell Plasticity in Tumor-Conditioned Skin: CD14(+) Cells at the Cross-Roads of Immune Activation and Suppression.Front Immunol. 2013 Nov 25;4:403. doi: 10.3389/fimmu.2013.00403. Front Immunol. 2013. PMID: 24324467 Free PMC article. Review.
Cited by
-
Glycan Sulfation Modulates Dendritic Cell Biology and Tumor Growth.Neoplasia. 2016 May;18(5):294-306. doi: 10.1016/j.neo.2016.04.004. Neoplasia. 2016. PMID: 27237321 Free PMC article.
-
Comparison the effects of two monocyte isolation methods, plastic adherence and magnetic activated cell sorting methods, on phagocytic activity of generated dendritic cells.Cell J. 2013 Fall;15(3):218-23. Epub 2013 Aug 24. Cell J. 2013. PMID: 24027662 Free PMC article.
-
Immunoadjuvant Activity of Fucoidans from the Brown Alga Fucus evanescens.Mar Drugs. 2020 Mar 11;18(3):155. doi: 10.3390/md18030155. Mar Drugs. 2020. PMID: 32168741 Free PMC article.
-
Vγ9γδ T Cell Induction by Human Umbilical Cord Blood Monocytes-Derived, Interferon-α-Stimulated Dendritic Cells.Cancer Control. 2020 Jan-Dec;27(1):1073274820974025. doi: 10.1177/1073274820974025. Cancer Control. 2020. PMID: 33222507 Free PMC article.
-
Isolation of dendritic cells from umbilical cord blood using magnetic activated cell sorting or adherence.Oncol Lett. 2015 Jul;10(1):67-70. doi: 10.3892/ol.2015.3198. Epub 2015 May 11. Oncol Lett. 2015. PMID: 26170978 Free PMC article.
References
-
- Mellman, I. Antigen processing and presentation by dendritic cells: cell biological mechanisms. Adv. Exp. Med. Biol. 2005;560:63–67. - PubMed
-
- Lipscomb, M.F., Masten, B.J. Dendritic cells: Immune regulators in health and disease. Physiol. Rev. 2002;82:97–130. - PubMed
-
- Bagheri, K., Delirezh, N., Moazzeni, S.M. PPDextract induces the maturation of human monocyte-derived dendritic cells. Immunopharmacol. Immunotoxicol. 2008;30:91–104. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
