Activity of 3-ketosteroid 9α-hydroxylase (KshAB) indicates cholesterol side chain and ring degradation occur simultaneously in Mycobacterium tuberculosis
- PMID: 21987574
- PMCID: PMC3220451
- DOI: 10.1074/jbc.M111.289975
Activity of 3-ketosteroid 9α-hydroxylase (KshAB) indicates cholesterol side chain and ring degradation occur simultaneously in Mycobacterium tuberculosis
Abstract
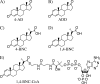
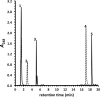
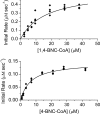
Mycobacterium tuberculosis (Mtb), a significant global pathogen, contains a cholesterol catabolic pathway. Although the precise role of cholesterol catabolism in Mtb remains unclear, the Rieske monooxygenase in this pathway, 3-ketosteroid 9α-hydroxylase (KshAB), has been identified as a virulence factor. To investigate the physiological substrate of KshAB, a rhodococcal acyl-CoA synthetase was used to produce the coenzyme A thioesters of two cholesterol derivatives: 3-oxo-23,24-bisnorchol-4-en-22-oic acid (forming 4-BNC-CoA) and 3-oxo-23,24-bisnorchola-1,4-dien-22-oic acid (forming 1,4-BNC-CoA). The apparent specificity constant (k(cat)/K(m)) of KshAB for the CoA thioester substrates was 20-30 times that for the corresponding 17-keto compounds previously proposed as physiological substrates. The apparent K(m)(O(2)) was 90 ± 10 μM in the presence of 1,4-BNC-CoA, consistent with the value for two other cholesterol catabolic oxygenases. The Δ(1) ketosteroid dehydrogenase KstD acted with KshAB to cleave steroid ring B with a specific activity eight times greater for a CoA thioester than the corresponding ketone. Finally, modeling 1,4-BNC-CoA into the KshA crystal structure suggested that the CoA moiety binds in a pocket at the mouth of the active site channel and could contribute to substrate specificity. These results indicate that the physiological substrates of KshAB are CoA thioester intermediates of cholesterol side chain degradation and that side chain and ring degradation occur concurrently in Mtb. This finding has implications for steroid metabolites potentially released by the pathogen during infection and for the design of inhibitors for cholesterol-degrading enzymes. The methodologies and rhodococcal enzymes used to generate thioesters will facilitate the further study of cholesterol catabolism.
Figures
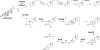
References
-
- WHO (2010) Global Tuberculosis Control: WHO Report 2010, pp. 2–42, World Health Organization, Geneva, Switzerland
-
- Russell D. G. (2007) Nat. Rev. Microbiol 5, 39–47 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

