Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT)
- PMID: 21987582
- PMCID: PMC3830549
- DOI: 10.1001/archophthalmol.2011.315
Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT)
Abstract
Objective: To determine whether there is a benefit in clinical outcomes with the use of topical corticosteroids as adjunctive therapy in the treatment of bacterial corneal ulcers.
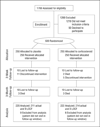
Methods: Randomized, placebo-controlled, double-masked, multicenter clinical trial comparing prednisolone sodium phosphate, 1.0%, to placebo as adjunctive therapy for the treatment of bacterial corneal ulcers. Eligible patients had a culture-positive bacterial corneal ulcer and received topical moxifloxacin for at least 48 hours before randomization.
Main outcome measures: The primary outcome was best spectacle-corrected visual acuity (BSCVA) at 3 months from enrollment. Secondary outcomes included infiltrate/scar size, reepithelialization, and corneal perforation.
Results: Between September 1, 2006, and February 22, 2010, 1769 patients were screened for the trial and 500 patients were enrolled. No significant difference was observed in the 3-month BSCVA (-0.009 logarithm of the minimum angle of resolution [logMAR]; 95% CI, -0.085 to 0.068; P = .82), infiltrate/scar size (P = .40), time to reepithelialization (P = .44), or corneal perforation (P > .99). A significant effect of corticosteroids was observed in subgroups of baseline BSCVA (P = .03) and ulcer location (P = .04). At 3 months, patients with vision of counting fingers or worse at baseline had 0.17 logMAR better visual acuity with corticosteroids (95% CI, -0.31 to -0.02; P = .03) compared with placebo, and patients with ulcers that were completely central at baseline had 0.20 logMAR better visual acuity with corticosteroids (-0.37 to -0.04; P = .02).
Conclusions: We found no overall difference in 3-month BSCVA and no safety concerns with adjunctive corticosteroid therapy for bacterial corneal ulcers.
Application to clinical practice: Adjunctive topical corticosteroid use does not improve 3-month vision in patients with bacterial corneal ulcers.
Trial registration: clinicaltrials.gov Identifier: NCT00324168.
Figures

References
-
- Cohen EJ. The case against the use of steroids in the treatment of bacterial keratitis. Arch Ophthalmol. 2009;127(1):103–104. - PubMed
-
- Hindman HB, Patel SB, Jun AS. Rationale for adjunctive topical corticosteroids in bacterial keratitis. Arch Ophthalmol. 2009;127(1):97–102. - PubMed
-
- Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99(2):226–231. - PubMed
-
- Girgis NI, Farid Z, Mikhail IA, Farrag I, Sultan Y, Kilpatrick ME. Dexamethasone treatment for bacterial meningitis in children and adults. Pediatr Infect Dis J. 1989;8(12):848–851. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

