The Protein Phosphatase 2A regulatory subunit Twins stabilizes Plk4 to induce centriole amplification
- PMID: 21987638
- PMCID: PMC3198173
- DOI: 10.1083/jcb.201107086
The Protein Phosphatase 2A regulatory subunit Twins stabilizes Plk4 to induce centriole amplification
Abstract
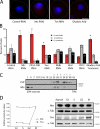
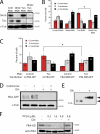
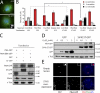
Centriole duplication is a tightly regulated process that must occur only once per cell cycle; otherwise, supernumerary centrioles can induce aneuploidy and tumorigenesis. Plk4 (Polo-like kinase 4) activity initiates centriole duplication and is regulated by ubiquitin-mediated proteolysis. Throughout interphase, Plk4 autophosphorylation triggers its degradation, thus preventing centriole amplification. However, Plk4 activity is required during mitosis for proper centriole duplication, but the mechanism stabilizing mitotic Plk4 is unknown. In this paper, we show that PP2A (Protein Phosphatase 2A(Twins)) counteracts Plk4 autophosphorylation, thus stabilizing Plk4 and promoting centriole duplication. Like Plk4, the protein level of PP2A's regulatory subunit, Twins (Tws), peaks during mitosis and is required for centriole duplication. However, untimely Tws expression stabilizes Plk4 inappropriately, inducing centriole amplification. Paradoxically, expression of tumor-promoting simian virus 40 small tumor antigen (ST), a reported PP2A inhibitor, promotes centrosome amplification by an unknown mechanism. We demonstrate that ST actually mimics Tws function in stabilizing Plk4 and inducing centriole amplification.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

