Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells
- PMID: 21987655
- PMCID: PMC3201195
- DOI: 10.1084/jem.20100977
Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells
Abstract
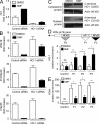
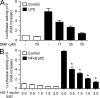
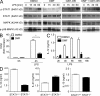
Fumarates improve multiple sclerosis (MS) and psoriasis, two diseases in which both IL-12 and IL-23 promote pathogenic T helper (Th) cell differentiation. However, both diseases show opposing responses to most established therapies. First, we show in humans that fumarate treatment induces IL-4-producing Th2 cells in vivo and generates type II dendritic cells (DCs) that produce IL-10 instead of IL-12 and IL-23. In mice, fumarates also generate type II DCs that induce IL-4-producing Th2 cells in vitro and in vivo and protect mice from experimental autoimmune encephalomyelitis. Type II DCs result from fumarate-induced glutathione (GSH) depletion, followed by increased hemoxygenase-1 (HO-1) expression and impaired STAT1 phosphorylation. Induced HO-1 is cleaved, whereupon the N-terminal fragment of HO-1 translocates into the nucleus and interacts with AP-1 and NF-κB sites of the IL-23p19 promoter. This interaction prevents IL-23p19 transcription without affecting IL-12p35, whereas STAT1 inactivation prevents IL-12p35 transcription without affecting IL-23p19. As a consequence, GSH depletion by small molecules such as fumarates induces type II DCs in mice and in humans that ameliorate inflammatory autoimmune diseases. This therapeutic approach improves Th1- and Th17-mediated autoimmune diseases such as psoriasis and MS by interfering with IL-12 and IL-23 production.
Figures

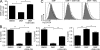
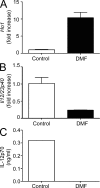


References
-
- Altmeyer P.J., Matthes U., Pawlak F., Hoffmann K., Frosch P.J., Ruppert P., Wassilew S.W., Horn T., Kreysel H.W., Lutz G., et al. 1994. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. J. Am. Acad. Dermatol. 30:977–981 10.1016/S0190-9622(94)70121-0 - DOI - PubMed
-
- Axtell R.C., de Jong B.A., Boniface K., van der Voort L.F., Bhat R., De Sarno P., Naves R., Han M., Zhong F., Castellanos J.G., et al. 2010. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 16:406–412 10.1038/nm.2110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

