Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis
- PMID: 21987776
- PMCID: PMC3189988
- DOI: 10.1093/infdis/jir326
Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis
Abstract
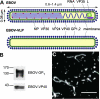
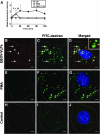
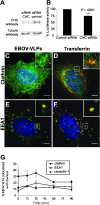
Virus entry into host cells is the first step of infection and a crucial determinant of pathogenicity. Here we show that Ebola virus-like particles (EBOV-VLPs) composed of the glycoprotein GP(1,2) and the matrix protein VP40 use macropinocytosis and clathrin-mediated endocytosis to enter cells. EBOV-VLPs applied to host cells induced actin-driven ruffling and enhanced FITC-dextran uptake, which indicated macropinocytosis as the main entry mechanism. This was further supported by inhibition of entry through inhibitors of actin polymerization (latrunculin A), Na(+)/H(+)-exchanger (EIPA), and PI3-kinase (wortmannin). A fraction of EBOV-VLPs, however, colocalized with clathrin heavy chain (CHC), and VLP uptake was reduced by CHC small interfering RNA transfection and expression of the dominant negative dynamin II-K44A mutant. In contrast, we found no evidence that EBOV-VLPs enter cells via caveolae. This work identifies macropinocytosis as the major, and clathrin-dependent endocytosis as an alternative, entry route for EBOV particles. Therefore, EBOV seems to utilize different entry pathways depending on both cell type and virus particle size.
Figures

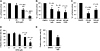
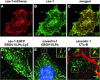
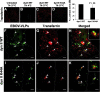
References
-
- Sanchez A, Geisbert TW, Feldmann H. Marburg and Ebola viruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007.
-
- Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: from discovery to vaccine. Nat Rev Immunol. 2003;3:677–85. - PubMed
-
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10:S110–21. - PubMed
-
- Schnittler HJ, Feldmann H. Marburg and Ebola hemorrhagic fevers: does the primary course of infection depend on the accessibility of organ-specific macrophages? Clin Infect Dis. 1998;27:404–6. - PubMed
-
- Feldmann H, Volchkov VE, Volchkova VA, Stroher U, Klenk HD. Biosynthesis and role of filoviral glycoproteins. J Gen Virol. 2001;82:2839–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

