Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload
- PMID: 21987816
- PMCID: PMC3203781
- DOI: 10.1073/pnas.1108559108
Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload
Abstract
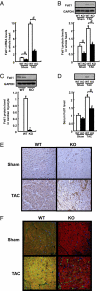
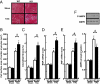
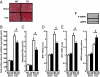
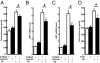
Factors secreted by the heart, referred to as "cardiokines," have diverse actions in the maintenance of cardiac homeostasis and remodeling. Follistatin-like 1 (Fstl1) is a secreted glycoprotein expressed in the adult heart and is induced in response to injurious conditions that promote myocardial hypertrophy and heart failure. The aim of this study was to investigate the role of cardiac Fstl1 in the remodeling response to pressure overload. Cardiac myocyte-specific Fstl1-KO mice were constructed and subjected to pressure overload induced by transverse aortic constriction (TAC). Although Fstl1-KO mice displayed no detectable baseline phenotype, TAC led to enhanced cardiac hypertrophic growth and a pronounced loss in ventricular performance by 4 wk compared with control mice. Conversely, mice that acutely or chronically overexpressed Fstl1 were resistant to pressure overload-induced hypertrophy and cardiac failure. Fstl1-deficient mice displayed a reduction in TAC-induced AMP-activated protein kinase (AMPK) activation in heart, whereas Fstl1 overexpression led to increased myocardial AMPK activation under these conditions. In cultured neonatal cardiomyocytes, administration of Fstl1 promoted AMPK activation and antagonized phenylephrine-induced hypertrophy. Inhibition of AMPK attenuated the antihypertrophic effect of Fstl1 treatment. These results document that cardiac Fstl1 functions as an autocrine/paracrine regulatory factor that antagonizes myocyte hypertrophic growth and the loss of ventricular performance in response to pressure overload, possibly through a mechanism involving the activation of the AMPK signaling axis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Follistatin-like 1 and its paralogs in heart development and cardiovascular disease.Heart Fail Rev. 2022 Nov;27(6):2251-2265. doi: 10.1007/s10741-022-10262-6. Epub 2022 Jul 22. Heart Fail Rev. 2022. PMID: 35867287 Free PMC article. Review.
-
Cardiac myocyte-specific ablation of follistatin-like 3 attenuates stress-induced myocardial hypertrophy.J Biol Chem. 2011 Mar 18;286(11):9840-8. doi: 10.1074/jbc.M110.197079. Epub 2011 Jan 18. J Biol Chem. 2011. PMID: 21245136 Free PMC article.
-
Cardiac myocyte-derived follistatin-like 1 prevents renal injury in a subtotal nephrectomy model.J Am Soc Nephrol. 2015 Mar;26(3):636-46. doi: 10.1681/ASN.2014020210. Epub 2014 Jul 28. J Am Soc Nephrol. 2015. PMID: 25071081 Free PMC article.
-
Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart.Circulation. 2008 Jun 17;117(24):3099-108. doi: 10.1161/CIRCULATIONAHA.108.767673. Epub 2008 Jun 2. Circulation. 2008. PMID: 18519848 Free PMC article.
-
Regulator of G-Protein Signaling 10 Negatively Regulates Cardiac Remodeling by Blocking Mitogen-Activated Protein Kinase-Extracellular Signal-Regulated Protein Kinase 1/2 Signaling.Hypertension. 2016 Jan;67(1):86-98. doi: 10.1161/HYPERTENSIONAHA.115.05957. Epub 2015 Nov 16. Hypertension. 2016. PMID: 26573707 Review.
Cited by
-
Genomic Binding Patterns of Forkhead Box Protein O1 Reveal Its Unique Role in Cardiac Hypertrophy.Circulation. 2020 Sep;142(9):882-898. doi: 10.1161/CIRCULATIONAHA.120.046356. Epub 2020 Jul 9. Circulation. 2020. PMID: 32640834 Free PMC article.
-
Genomic, Proteomic, and Metabolic Comparisons of Small Animal Models of Heart Failure With Preserved Ejection Fraction: A Tale of Mice, Rats, and Cats.J Am Heart Assoc. 2022 Aug 2;11(15):e026071. doi: 10.1161/JAHA.122.026071. Epub 2022 Jul 29. J Am Heart Assoc. 2022. PMID: 35904190 Free PMC article. Review.
-
Follistatin-like 1 and its paralogs in heart development and cardiovascular disease.Heart Fail Rev. 2022 Nov;27(6):2251-2265. doi: 10.1007/s10741-022-10262-6. Epub 2022 Jul 22. Heart Fail Rev. 2022. PMID: 35867287 Free PMC article. Review.
-
Gene expression analysis to identify mechanisms underlying heart failure susceptibility in mice and humans.Basic Res Cardiol. 2017 Dec 29;113(1):8. doi: 10.1007/s00395-017-0666-6. Print 2018 Jan 8. Basic Res Cardiol. 2017. PMID: 29288409 Free PMC article.
-
Natural genetic variation of the cardiac transcriptome in non-diseased donors and patients with dilated cardiomyopathy.Genome Biol. 2017 Sep 14;18(1):170. doi: 10.1186/s13059-017-1286-z. Genome Biol. 2017. PMID: 28903782 Free PMC article.
References
-
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. - PubMed
-
- Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. - PubMed
-
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: A new therapeutic target? Circulation. 2004;109:1580–1589. - PubMed
-
- Frost RJ, Engelhardt S. A secretion trap screen in yeast identifies protease inhibitor 16 as a novel antihypertrophic protein secreted from the heart. Circulation. 2007;116:1768–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

