PPARs and lipid ligands in inflammation and metabolism
- PMID: 21988241
- PMCID: PMC3437919
- DOI: 10.1021/cr2001355
PPARs and lipid ligands in inflammation and metabolism
Figures
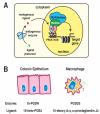



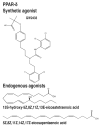
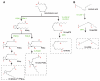
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

