Reported paediatric adverse drug reactions in the UK 2000-2009
- PMID: 21988288
- PMCID: PMC3370348
- DOI: 10.1111/j.1365-2125.2011.04113.x
Reported paediatric adverse drug reactions in the UK 2000-2009
Abstract
Aims: The UK Medicines and Healthcare products Regulatory Agency (MHRA) runs a national spontaneous reporting system (Yellow Card Scheme) to collect 'suspected' adverse drug reaction (ADR) data. MHRA advice is to report all suspected ADRs in paediatric (<17 years) patients.
Methods: Data on all ADRs reported to the MHRA in patients <17 years from the years 2000-9 were supplied in two datasets, inclusive and exclusive of vaccines.
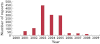
Results: Of 222 755 ADR reports received by the MHRA from 2000-9, 31726 (14.2%) were in children <17 years. The number of reports in 2000 was greater than in subsequent years (12035) due to a national vaccination programme (Meningococcal Serogroup C conjugate vaccine). The median number of ADR reports per annum (2001-2009) for children was 2146 (95% CI 1801, 2575). Vaccines were included in 22102 (66.5%) paediatric ADR reports, with Meningococcal Serogroup C conjugate vaccine reported most frequently (12106 reports) and headache the commonest symptom (3163). Excluding vaccines, methylphenidate (653 reports) and atomoxetine (491) were the most commonly reported medications, and the most commonly reported symptom was vomiting (374). Reporting by nurses increased from 396 in 2001 to 1295 in 2009 (41.8% of all reports); reporting by doctors stayed constant. Reports from patients, parents or carers more than doubled but remained infrequent (1.5% in 2005, 4.0% in 2009).
Conclusions: Although under-reporting is probably common, the Yellow Card Scheme in the UK receives more than 2000 reports per year on patients <17 years. Nurses now report more suspected ADRs in children than any other healthcare professional.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
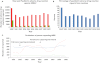
 ) Total number; (
) Total number; ( ) Total number < 17 years; (C) (
) Total number < 17 years; (C) ( ) Doctors; (
) Doctors; ( ) Nurses; (
) Nurses; ( ) Pharmacist; (
) Pharmacist; ( ) Parent/Carer
) Parent/Carer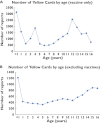
References
-
- Office for National Statistics. Key Population and Vital Statistics. Key Population and Vital Statistics No 34, PPI No 30, 2007 Data: population and vital statistics by area of usual residence in the United Kingdom, 2007. 2009. Available at http://www.ons.gov.uk/ons/rel/kpvs/key-population-and-vital-statistics/n... (last accessed 31 October 2011)
-
- Office for National Statistics. Prescriptions dispensed in the community statistics for 1997 to 2007: England. 2008. Available at http://www.ic.nhs.uk/webfiles/publications/PCA%20publication/Final%20ver... (last accessed 17 May 2011)
-
- Shirkey H. Editorial comment: therapeutic orphans. J Pediatr. 1968;72:119–20. - PubMed
-
- Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999;88:965–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

