The effect of nano-scale topography on keratinocyte phenotype and wound healing following burn injury
- PMID: 21988618
- PMCID: PMC3313614
- DOI: 10.1089/ten.TEA.2011.0307
The effect of nano-scale topography on keratinocyte phenotype and wound healing following burn injury
Abstract
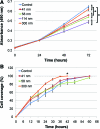
Topographic modulation of tissue response is an important consideration in the design and manufacture of a biomaterial. In developing new tissue therapies for skin, all levels of architecture, including the nanoscale need to be considered. Here we show that keratinocyte phenotype is affected by nanoscale changes in topography with cell morphology, proliferation, and migration influenced by the pore size in anodic aluminum oxide membranes. A membrane with a pore size of 300 nm, which enhanced cell phenotype in vitro, was used as a dressing to cover a partial thickness burn injury in the pig. Wounds dressed with the membrane showed evidence of advanced healing with significantly less organizing granulation tissue and more mature epidermal layers than control wounds dressed with a standard burns dressing. The results demonstrate the importance of nanoscale topography in modulating keratinocyte phenotype and skin wound healing.
Figures





Similar articles
-
The potential of nanoporous anodic aluminium oxide membranes to influence skin wound repair.Tissue Eng Part A. 2009 Dec;15(12):3753-63. doi: 10.1089/ten.TEA.2008.0594. Tissue Eng Part A. 2009. PMID: 19527180
-
[Clinical study of cell sheets containing allogeneic keratinocytes and fibroblasts for the treatment of partial-thickness burn wounds].Zhonghua Shao Shang Za Zhi. 2020 Mar 20;36(3):171-178. doi: 10.3760/cma.j.cn501120-20191113-00426. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32241042 Chinese.
-
Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration.Theranostics. 2020 Aug 8;10(22):9970-9983. doi: 10.7150/thno.46639. eCollection 2020. Theranostics. 2020. PMID: 32929328 Free PMC article.
-
Natural and synthetic polymers for wounds and burns dressing.Int J Pharm. 2014 Mar 25;463(2):127-36. doi: 10.1016/j.ijpharm.2013.12.015. Epub 2013 Dec 22. Int J Pharm. 2014. PMID: 24368109 Review.
-
Re-epithelialization. Human keratinocyte locomotion.Dermatol Clin. 1993 Oct;11(4):641-6. Dermatol Clin. 1993. PMID: 8222348 Review.
Cited by
-
The influence of substrate topography on the migration of corneal epithelial wound borders.Biomaterials. 2013 Dec;34(37):9244-51. doi: 10.1016/j.biomaterials.2013.08.042. Epub 2013 Sep 7. Biomaterials. 2013. PMID: 24016856 Free PMC article.
-
Review of Integrin-Targeting Biomaterials in Tissue Engineering.Adv Healthc Mater. 2020 Dec;9(23):e2000795. doi: 10.1002/adhm.202000795. Epub 2020 Sep 16. Adv Healthc Mater. 2020. PMID: 32940020 Free PMC article. Review.
-
Biochemical and Biophysical Cues in Matrix Design for Chronic and Diabetic Wound Treatment.Tissue Eng Part B Rev. 2017 Feb;23(1):9-26. doi: 10.1089/ten.TEB.2016.0200. Epub 2016 Aug 19. Tissue Eng Part B Rev. 2017. PMID: 27405960 Free PMC article.
-
Biomaterials for Skin Substitutes.Adv Healthc Mater. 2018 Mar;7(5):10.1002/adhm.201700897. doi: 10.1002/adhm.201700897. Epub 2017 Dec 22. Adv Healthc Mater. 2018. PMID: 29271580 Free PMC article. Review.
-
Effect of collagen nanotopography on keloid fibroblast proliferation and matrix synthesis: implications for dermal wound healing.Tissue Eng Part A. 2014 Oct;20(19-20):2728-36. doi: 10.1089/ten.TEA.2013.0539. Epub 2014 May 20. Tissue Eng Part A. 2014. PMID: 24724556 Free PMC article.
References
-
- Chou L. Firth J.D. Uitto V.-J. Brunette D.M. Substratum surface topography alters cell shape and regulates fibronectin mRNA level, mRNA stability, secretion and assembly in human fibroblasts. J Cell Sci. 1995;108:1563. - PubMed
-
- Curtis A. Wilkinson C. Topographical control of cells. Biomaterials. 1997;18:1573. - PubMed
-
- Zeltinger J. Sherwood J.K. Graham D.A. Mueller R. Griffith L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557. - PubMed
-
- O'Brien F.J. Harley B.A. Yannas I.V. Gibson L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433. - PubMed
-
- McMillan J.R. Akiyama M. Tanaka M. Yamamoto S. Goto M. Abe R. Sawamura D. Shimomura M. Shimizu H. Small-diameter porous poly (epsilon-caprolactone) films enhance adhesion and growth of human cultured epidermal keratinocyte and dermal fibroblast cells. Tissue Eng. 2007;13:789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

