The mechanisms by which polyamines accelerate tumor spread
- PMID: 21988863
- PMCID: PMC3206444
- DOI: 10.1186/1756-9966-30-95
The mechanisms by which polyamines accelerate tumor spread
Abstract
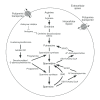
Increased polyamine concentrations in the blood and urine of cancer patients reflect the enhanced levels of polyamine synthesis in cancer tissues arising from increased activity of enzymes responsible for polyamine synthesis. In addition to their de novo polyamine synthesis, cells can take up polyamines from extracellular sources, such as cancer tissues, food, and intestinal microbiota. Because polyamines are indispensable for cell growth, increased polyamine availability enhances cell growth. However, the malignant potential of cancer is determined by its capability to invade to surrounding tissues and metastasize to distant organs. The mechanisms by which increased polyamine levels enhance the malignant potential of cancer cells and decrease anti-tumor immunity are reviewed. Cancer cells with a greater capability to synthesize polyamines are associated with increased production of proteinases, such as serine proteinase, matrix metalloproteinases, cathepsins, and plasminogen activator, which can degrade surrounding tissues. Although cancer tissues produce vascular growth factors, their deregulated growth induces hypoxia, which in turn enhances polyamine uptake by cancer cells to further augment cell migration and suppress CD44 expression. Increased polyamine uptake by immune cells also results in reduced cytokine production needed for anti-tumor activities and decreases expression of adhesion molecules involved in anti-tumor immunity, such as CD11a and CD56. Immune cells in an environment with increased polyamine levels lose anti-tumor immune functions, such as lymphokine activated killer activities. Recent investigations revealed that increased polyamine availability enhances the capability of cancer cells to invade and metastasize to new tissues while diminishing immune cells' anti-tumor immune functions.
Figures

References
-
- Durie BG, Salmon SE, Russell DH. Polyamines as markers of response and disease activity in cancer chemotherapy. Cancer Res. 1977;37:214–221. - PubMed
-
- Loser C, Folsch UR, Paprotny C, Creutzfeldt W. Polyamines in colorectal cancer. Evaluation of polyamine concentrations in the colon tissue, serum, and urine of 50 patients with colorectal cancer. Cancer. 1990;65:958–966. doi: 10.1002/1097-0142(19900215)65:4<958::AID-CNCR2820650423>3.0.CO;2-Z. - DOI - PubMed
-
- Chatel M, Darcel F, Quemener V, Hercouet H, Moulinoux JP. Red blood cell polyamines as biochemical markers of supratentorial malignant gliomas. Anticancer Res. 1987;7:33–38. - PubMed
-
- Kubota S, Okada M, Yoshimoto M, Murata N, Yamasaki Z, Wada T, Imahori K, Ohsawa N, Takaku F. Urinary polyamines as a tumor marker. Cancer Detect Prev. 1985;8:189–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

