Gut triglyceride production
- PMID: 21989069
- PMCID: PMC3319358
- DOI: 10.1016/j.bbalip.2011.09.013
Gut triglyceride production
Abstract
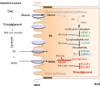
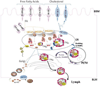
Our knowledge of how the body absorbs triacylglycerols (TAG) from the diet and how this process is regulated has increased at a rapid rate in recent years. Dietary TAG are hydrolyzed in the intestinal lumen to free fatty acids (FFA) and monoacylglycerols (MAG), which are taken up by enterocytes from their apical side, transported to the endoplasmic reticulum (ER) and resynthesized into TAG. TAG are assembled into chylomicrons (CM) in the ER, transported to the Golgi via pre-chylomicron transport vesicles and secreted towards the basolateral side. In this review, we mainly focus on the roles of key proteins involved in uptake and intracellular transport of fatty acids, their conversion to TAG and packaging into CM. We will also discuss intracellular transport and secretion of CM. Moreover, we will bring to light few factors that regulate gut triglyceride production. Furthermore, we briefly summarize pathways involved in cholesterol absorption. This article is part of a Special Issue entitled Triglyceride Metabolism and Disease.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
References
-
- Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 1993;268:17665. - PubMed
-
- Adeli K, Lewis GF. Intestinal lipoprotein overproduction in insulin-resistant states. Curr. Opin. Lipidol. 2008;19:221. - PubMed
-
- Agellon LB, Drozdowski L, Li L, Iordache C, Luong L, Clandinin MT, Uwiera RR, Toth MJ, Thomson AB. Loss of intestinal fatty acid binding protein increases the susceptibility of male mice to high fat diet-induced fatty liver. Biochim. Biophys. Acta. 2007;1771:1283. - PubMed
-
- Alpers DH, Bass NM, Engle MJ, Schryver-Kecskemeti K. Intestinal fatty acid binding protein may favor differential apical fatty acid binding in the intestine. Biochim. Biophys. Acta. 2000;1483:352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

