Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development
- PMID: 21989106
- PMCID: PMC3207869
- DOI: 10.1186/1749-8104-6-33
Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development
Abstract
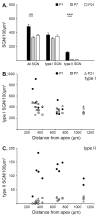
Background: The mechanisms that consolidate neural circuitry are a major focus of neuroscience. In the mammalian cochlea, the refinement of spiral ganglion neuron (SGN) innervation to the inner hair cells (by type I SGNs) and the outer hair cells (by type II SGNs) is accompanied by a 25% loss of SGNs.
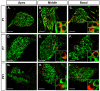
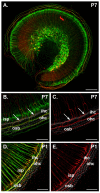
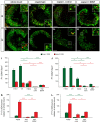
Results: We investigated the segregation of neuronal loss in the mouse cochlea using β-tubulin and peripherin antisera to immunolabel all SGNs and selectively type II SGNs, respectively, and discovered that it is the type II SGN population that is predominately lost within the first postnatal week. Developmental neuronal loss has been attributed to the decline in neurotrophin expression by the target hair cells during this period, so we next examined survival of SGN sub-populations using tissue culture of the mid apex-mid turn region of neonatal mouse cochleae. In organotypic culture for 48 hours from postnatal day 1, endogenous trophic support from the organ of Corti proved sufficient to maintain all type II SGNs; however, a large proportion of type I SGNs were lost. Culture of the spiral ganglion as an explant, with removal of the organ of Corti, led to loss of the majority of both SGN sub-types. Brain-derived neurotrophic factor (BDNF) added as a supplement to the media rescued a significant proportion of the SGNs, particularly the type II SGNs, which also showed increased neuritogenesis. The known decline in BDNF production by the rodent sensory epithelium after birth is therefore a likely mediator of type II neuron apoptosis.
Conclusion: Our study thus indicates that BDNF supply from the organ of Corti supports consolidation of type II innervation in the neonatal mouse cochlea. In contrast, type I SGNs likely rely on additional sources for trophic support.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

