Survival endpoints in colorectal cancer and the effect of second primary other cancer on disease free survival
- PMID: 21989154
- PMCID: PMC3209454
- DOI: 10.1186/1471-2407-11-438
Survival endpoints in colorectal cancer and the effect of second primary other cancer on disease free survival
Abstract
Background: In cancer research the selection and definitions of survival endpoints are important and yet they are not used consistently. The aim of this study was to compare different survival endpoints in patients with primary colorectal cancer (CRC) and to understand the effect of second primary other cancer on disease-free survival (DFS) calculations.
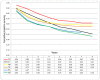
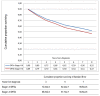
Methods: A population-based cohort of 415 patients with CRC, 332 of whom were treated with curative intention between the years 2000-2003, was analysed. Events such as locoregional recurrence, distant metastases, second primary cancers, death, cause of death and loss to follow-up were recorded. Different survival endpoints, including DFS, overall survival, cancer-specific survival, relapse-free survival, time to treatment failure and time to recurrence were compared and DFS was calculated with and without inclusion of second primary other cancers.
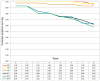
Results: The events that occurred most often in patients treated with curative intention were non-cancer-related death (n = 74), distant metastases (n = 66) and death from CRC (n = 59). DFS was the survival endpoint with most events (n = 170) followed by overall survival (n = 144) and relapse-free survival (n = 139). Fewer events were seen for time to treatment failure (n = 80), time to recurrence (n = 68) and cancer-specific survival (n = 59). Second primary other cancer occurred in 26 patients and its inclusion as an event in DFS calculations had a detrimental effect on the survival. The DFS for patients with stage I-III disease was 62% after 5 years if second primary other cancer was not included as an event, compared with 58% if it was. However, the difference was larger for stage II (68 vs 60%) than for stage III (49 vs 47%).
Conclusions: The inclusion of second primary other cancer as an endpoint in DFS analyses significantly alters the DFS for patients with CRC. Researchers and journals must clearly define survival endpoints in all trial protocols and published manuscripts.
Figures



References
-
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. - PubMed
-
- Punt CJ, Buyse M, Kohne CH, Hohenberger P, Labianca R, Schmoll HJ, Pahlman L, Sobrero A, Douillard JY. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007;99(13):998–1003. doi: 10.1093/jnci/djm024. - DOI - PubMed
-
- Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O'Callaghan CJ, Francini G, Grothey A, O'Connell M, Catalano PJ, Blanke CD, Kerr D, Green E, Wolmark N, Andre T, Goldberg RM, De Gramont A. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664–8670. doi: 10.1200/JCO.2005.01.6071. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

