Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study
- PMID: 21989407
- PMCID: PMC3203079
- DOI: 10.1186/1471-2458-11-786
Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study
Abstract
Background: Cigarette smoking is a tough addiction to break. Therefore, improved approaches to smoking cessation are necessary. The electronic-cigarette (e-Cigarette), a battery-powered electronic nicotine delivery device (ENDD) resembling a cigarette, may help smokers to remain abstinent during their quit attempt or to reduce cigarette consumption. Efficacy and safety of these devices in long-term smoking cessation and/or smoking reduction studies have never been investigated.
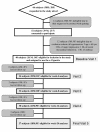
Methods: In this prospective proof-of-concept study we monitored possible modifications in smoking habits of 40 regular smokers (unwilling to quit) experimenting the 'Categoria' e-Cigarette with a focus on smoking reduction and smoking abstinence. Study participants were invited to attend a total of five study visits: at baseline, week-4, week-8, week-12 and week-24. Product use, number of cigarettes smoked, and exhaled carbon monoxide (eCO) levels were measured at each visit. Smoking reduction and abstinence rates were calculated. Adverse events and product preferences were also reviewed.
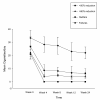
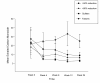
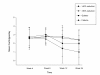
Results: Sustained 50% reduction in the number of cig/day at week-24 was shown in 13/40(32.5%) participants; their median of 25 cigs/day decreasing to 6 cigs/day (p < 0.001). Sustained 80% reduction was shown in 5/40(12.5%) participants; their median of 30 cigs/day decreasing to 3 cigs/day (p = 0.043). Sustained smoking abstinence at week-24 was observed in 9/40(22.5%) participants, with 6/9 still using the e-Cigarette by the end of the study. Combined sustained 50% reduction and smoking abstinence was shown in 22/40 (55%) participants, with an overall 88% fall in cigs/day. Mouth (20.6%) and throat (32.4%) irritation, and dry cough (32.4%) were common, but diminished substantially by week-24. Overall, 2 to 3 cartridges/day were used throughout the study. Participants' perception and acceptance of the product was good.
Conclusion: The use of e-Cigarette substantially decreased cigarette consumption without causing significant side effects in smokers not intending to quit (http://ClinicalTrials.gov number NCT01195597).
Figures
References
-
- Tobacco or Health:a Global Status Report. Geneva; 1997.
-
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. - DOI - PMC - PubMed
-
- Boyle P, Gray N, Henningfield J, Seffrin J, Zatonski W. Tobacco and Public Health: Science and Policy. Oxford: Oxford University Press; 2004.
-
- Services UDoHaH: USA, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, editor. The health benefits of smoking cessation. 1990.
-
- Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997;96(4):1089–1096. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

