Seeking-taking chain schedules of cocaine and sucrose self-administration: effects of reward size, reward omission, and α-flupenthixol
- PMID: 21989807
- PMCID: PMC3313030
- DOI: 10.1007/s00213-011-2525-8
Seeking-taking chain schedules of cocaine and sucrose self-administration: effects of reward size, reward omission, and α-flupenthixol
Abstract
Rationale: In heterogeneous seeking-taking (ST) chain schedules of self-administration, seeking rewards and taking rewards are distinct actions, giving animals explicit control over their intake of the reward. However, the neurobehavioral characteristics of ST chain schedules are relatively unexplored.
Objectives: This study was made to evaluate two variants of ST chain schedules of self-administration to measure seeking and taking of sucrose and cocaine in rats.
Methods: Rats had to respond on one lever (seeking lever) under a random interval (RI) or under a progressive ratio (PR) schedule, to gain access to a second lever (taking lever), responding on which under a fixed-ratio 1 (FR-1) schedule of reinforcement delivered the reward. We assessed the effects of reward size, reward omission, and administration of the dopamine receptor antagonist α-flupenthixol. The effects of α-flupenthixol on responding for cocaine or sucrose under an FR-1 schedule of reinforcement were also assessed.
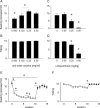
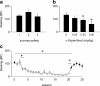
Results: Cocaine seeking under both schedules was reduced by decreasing reward size, reward omission, and α-flupenthixol treatment. Cocaine taking was decreased by α-flupenthixol treatment and reward omission, but not by altering reward size. Sucrose seeking was not affected by reward size, but was reduced by α-flupenthixol and reward omission. Sucrose taking was diminished by reward omission only. α-Flupenthixol increased cocaine but not sucrose intake under an FR-1 schedule of reinforcement.
Conclusions: Both ST(PR) and ST(RI) schedules can be used to assess seeking and taking of sucrose and cocaine. Dopaminergic neurotransmission mediates the positive subjective properties of cocaine but not sucrose and the motivational properties of both sucrose and cocaine.
Figures



Similar articles
-
Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine.Neuropsychopharmacology. 2012 Jan;37(2):487-98. doi: 10.1038/npp.2011.209. Epub 2011 Sep 14. Neuropsychopharmacology. 2012. PMID: 21918505 Free PMC article.
-
Distinct contributions of dopamine receptors in the nucleus accumbens core or shell to established cocaine reinforcement under a second-order schedule.Eur Neuropsychopharmacol. 2008 Dec;18(12):888-96. doi: 10.1016/j.euroneuro.2008.07.007. Epub 2008 Aug 28. Eur Neuropsychopharmacol. 2008. PMID: 18760571
-
Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking.Neuropsychopharmacology. 2012 Oct;37(11):2456-66. doi: 10.1038/npp.2012.104. Epub 2012 Jun 27. Neuropsychopharmacology. 2012. PMID: 22739470 Free PMC article.
-
Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations.Neurosci Biobehav Rev. 2013 Nov;37(9 Pt A):2026-36. doi: 10.1016/j.neubiorev.2013.04.017. Epub 2013 May 10. Neurosci Biobehav Rev. 2013. PMID: 23669047 Free PMC article. Review.
-
Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour.Psychopharmacology (Berl). 2000 Dec;153(1):17-30. doi: 10.1007/s002130000566. Psychopharmacology (Berl). 2000. PMID: 11255926 Review.
Cited by
-
Deep brain stimulation reveals a dissociation of consummatory and motivated behaviour in the medial and lateral nucleus accumbens shell of the rat.PLoS One. 2012;7(3):e33455. doi: 10.1371/journal.pone.0033455. Epub 2012 Mar 13. PLoS One. 2012. PMID: 22428054 Free PMC article.
-
Disrupted social development enhances the motivation for cocaine in rats.Psychopharmacology (Berl). 2014 Apr;231(8):1695-704. doi: 10.1007/s00213-013-3362-8. Epub 2013 Dec 6. Psychopharmacology (Berl). 2014. PMID: 24311358 Free PMC article.
-
Impact of Sex and Gonadal Hormones on Cocaine and Food Reinforcement Paradigms.J Addict Res Ther. 2011 Dec 20;S4(2):2963. doi: 10.4172/2155-6105.s4-002. Epub 2011 Dec 15. J Addict Res Ther. 2011. PMID: 22545233 Free PMC article.
-
Phasic mesolimbic dopamine signaling precedes and predicts performance of a self-initiated action sequence task.Biol Psychiatry. 2012 May 15;71(10):846-54. doi: 10.1016/j.biopsych.2011.12.019. Epub 2012 Feb 2. Biol Psychiatry. 2012. PMID: 22305286 Free PMC article.
-
Increased elasticity of sucrose demand during hyperdopaminergic states in rats.Psychopharmacology (Berl). 2022 Mar;239(3):773-794. doi: 10.1007/s00213-022-06068-x. Epub 2022 Jan 31. Psychopharmacology (Berl). 2022. PMID: 35102422 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

