Inhibition of gluconeogenic genes by calcium-regulated heat-stable protein 1 via repression of peroxisome proliferator-activated receptor α
- PMID: 21990353
- PMCID: PMC3220516
- DOI: 10.1074/jbc.M111.232918
Inhibition of gluconeogenic genes by calcium-regulated heat-stable protein 1 via repression of peroxisome proliferator-activated receptor α
Abstract
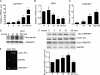
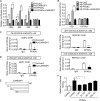
Gluconeogenesis contributes to insulin resistance in type 1 and type 2 diabetes, but its regulation and the underlying molecular mechanisms remain unclear. Recently, calcium-regulated heat-stable protein 1 (CARHSP1) was identified as a biomarker for diabetic complications. In this study, we investigated the role of CARHSP1 in hepatic gluconeogenesis. We assessed the regulation of hepatic CARHSP1 expression under conditions of fasting and refeeding. Adenovirus-mediated CARHSP1 overexpression and siRNA-mediated knockdown experiments were performed to characterize the role of CARHSP1 in the regulation of gluconeogenic gene expression. Here, we document for the first time that CARHSP1 is regulated by nutrient status in the liver and functions at the transcriptional level to negatively regulate gluconeogenic genes, including the glucose-6-phosphatase catalytic subunit (G6Pc) and phosphoenolpyruvate carboxykinase 1 (PEPCK1). In addition, we found that CARHSP1 can physically interact with peroxisome proliferator-activated receptor-α (PPARα) and inhibit its transcriptional activity. Both pharmacological and genetic ablations of PPARα attenuate the inhibitory effect of CARHSP1 on gluconeogenic gene expression in hepatocytes. Our data suggest that CARHSP1 inhibits hepatic gluconeogenic gene expression via repression of PPARα and that CARHSP1 may be a molecular target for the treatment of diabetes.
Figures


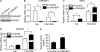
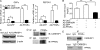


References
-
- Boden G., Chen X., Stein T. P. (2001) Am. J. Physiol. Endocrinol. Metab. 280, E23–30 - PubMed
-
- Perriello G., Pampanelli S., Del Sindaco P., Lalli C., Ciofetta M., Volpi E., Santeusanio F., Brunetti P., Bolli G. B. (1997) Diabetes 46, 1010–1016 - PubMed
-
- Wajngot A., Chandramouli V., Schumann W. C., Ekberg K., Jones P. K., Efendic S., Landau B. R. (2001) Metabolism 50, 47–52 - PubMed
-
- Biddinger S. B., Kahn C. R. (2006) Annu. Rev. Physiol. 68, 123–158 - PubMed
-
- Quinn P. G., Yeagley D. (2005) Curr. Drug Targets Immune Endocr. Metabol. Disord. 5, 423–437 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

